Guanine nucleotide exchange factor Epac2-dependent activation of the GTP-binding protein Rap2A mediates cAMP-dependent growth arrest in neuroendocrine cells
- PMID: 28546426
- PMCID: PMC5519371
- DOI: 10.1074/jbc.M117.790329
Guanine nucleotide exchange factor Epac2-dependent activation of the GTP-binding protein Rap2A mediates cAMP-dependent growth arrest in neuroendocrine cells
Abstract
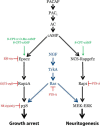
First messenger-dependent activation of MAP kinases in neuronal and endocrine cells is critical for cell differentiation and function and requires guanine nucleotide exchange factor (GEF)-mediated activation of downstream Ras family small GTPases, which ultimately lead to ERK, JNK, and p38 phosphorylation. Because there are numerous GEFs and also a host of Ras family small GTPases, it is important to know which specific GEF-small GTPase dyad functions in a given cellular process. Here we investigated the upstream activators and downstream effectors of signaling via the GEF Epac2 in the neuroendocrine NS-1 cell line. Three cAMP sensors, Epac2, PKA, and neuritogenic cAMP sensor-Rapgef2, mediate distinct cellular outputs: p38-dependent growth arrest, cAMP response element-binding protein-dependent cell survival, and ERK-dependent neuritogenesis, respectively, in these cells. Previously, we found that cAMP-induced growth arrest of PC12 and NS-1 cells requires Epac2-dependent activation of p38 MAP kinase, which posed the important question of how Epac2 engages p38 without simultaneously activating other MAP kinases in neuronal and endocrine cells. We now show that the small GTP-binding protein Rap2A is the obligate effector for, and GEF substrate of, Epac2 in mediating growth arrest through p38 activation in NS-1 cells. This new pathway is distinctly parcellated from the G protein-coupled receptor → Gs → adenylate cyclase → cAMP → PKA → cAMP response element-binding protein pathway mediating cell survival and the G protein-coupled receptor → Gs → adenylate cyclase → cAMP → neuritogenic cAMP sensor-Rapgef2 → B-Raf → MEK → ERK pathway mediating neuritogenesis in NS-1 cells.
Keywords: ERK; cAMP; guanine nucleotide exchange factor (GEF); p38; p38 MAPK; small GTPase.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures









Similar articles
-
Separate cyclic AMP sensors for neuritogenesis, growth arrest, and survival of neuroendocrine cells.J Biol Chem. 2014 Apr 4;289(14):10126-39. doi: 10.1074/jbc.M113.529321. Epub 2014 Feb 24. J Biol Chem. 2014. PMID: 24567337 Free PMC article.
-
Cyclic Adenosine 3',5'-Monophosphate Elevation and Biological Signaling through a Secretin Family Gs-Coupled G Protein-Coupled Receptor Are Restricted to a Single Adenylate Cyclase Isoform.Mol Pharmacol. 2015 Jun;87(6):928-35. doi: 10.1124/mol.115.098087. Epub 2015 Mar 13. Mol Pharmacol. 2015. PMID: 25769305 Free PMC article.
-
Rapgef2 connects GPCR-mediated cAMP signals to ERK activation in neuronal and endocrine cells.Sci Signal. 2013 Jun 25;6(281):ra51. doi: 10.1126/scisignal.2003993. Sci Signal. 2013. PMID: 23800469 Free PMC article.
-
Rit subfamily small GTPases: regulators in neuronal differentiation and survival.Cell Signal. 2013 Oct;25(10):2060-8. doi: 10.1016/j.cellsig.2013.06.002. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770287 Free PMC article. Review.
-
Structure and functional roles of Epac2 (Rapgef4).Gene. 2016 Jan 10;575(2 Pt 3):577-83. doi: 10.1016/j.gene.2015.09.029. Epub 2015 Sep 24. Gene. 2016. PMID: 26390815 Free PMC article. Review.
Cited by
-
Sodium current inhibition following stimulation of exchange protein directly activated by cyclic-3',5'-adenosine monophosphate (Epac) in murine skeletal muscle.Sci Rep. 2019 Feb 13;9(1):1927. doi: 10.1038/s41598-018-36386-0. Sci Rep. 2019. PMID: 30760734 Free PMC article.
-
Cocaine-Dependent Acquisition of Locomotor Sensitization and Conditioned Place Preference Requires D1 Dopaminergic Signaling through a Cyclic AMP, NCS-Rapgef2, ERK, and Egr-1/Zif268 Pathway.J Neurosci. 2021 Jan 27;41(4):711-725. doi: 10.1523/JNEUROSCI.1497-20.2020. Epub 2020 Dec 2. J Neurosci. 2021. PMID: 33268547 Free PMC article.
-
Application of Machine Learning and Weighted Gene Co-expression Network Algorithm to Explore the Hub Genes in the Aging Brain.Front Aging Neurosci. 2021 Oct 18;13:707165. doi: 10.3389/fnagi.2021.707165. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34733151 Free PMC article.
-
Krüppel-Like Factors 9 and 13 Block Axon Growth by Transcriptional Repression of Key Components of the cAMP Signaling Pathway.Front Mol Neurosci. 2020 Nov 12;13:602638. doi: 10.3389/fnmol.2020.602638. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33281552 Free PMC article.
-
Inhibition of Epac2 Attenuates Neural Cell Apoptosis and Improves Neurological Deficits in a Rat Model of Traumatic Brain Injury.Front Neurosci. 2018 Apr 23;12:263. doi: 10.3389/fnins.2018.00263. eCollection 2018. Front Neurosci. 2018. PMID: 29740274 Free PMC article.
References
-
- Overbeck A. F., Brtva T. R., Cox A. D., Graham S. M., Huff S. Y., Khosravi-Far R., Quilliam L. A., Solski P. A., and Der C. J. (1995) Guanine nucleotide exchange factors: activators of Ras superfamily proteins. Mol. Reprod. Dev. 42, 468–476 - PubMed
-
- Stork P. J. (2003) Does Rap1 deserve a bad Rap? Trends Biochem. Sci. 28, 267–275 - PubMed
-
- Murphy L. O., and Blenis J. (2006) MAPK signal specificity: the right place at the right time. Trends Biochem. Sci. 31, 268–275 - PubMed
-
- Quilliam L. A., Castro A. F., Rogers-Graham K. S., Martin C. B., Der C. J., and Bi C. (1999) M-Ras/R-Ras3, a transforming ras protein regulated by Sos1, GRF1, and p120 Ras GTPase-activating protein, interacts with the putative Ras effector AF6. J. Biol. Chem. 274, 23850–23857 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

