Behavioral Deficits Following Withdrawal from Chronic Ethanol Are Influenced by SLO Channel Function in Caenorhabditis elegans
- PMID: 28546434
- PMCID: PMC5500142
- DOI: 10.1534/genetics.116.193102
Behavioral Deficits Following Withdrawal from Chronic Ethanol Are Influenced by SLO Channel Function in Caenorhabditis elegans
Abstract
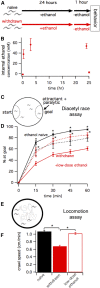
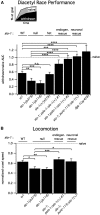
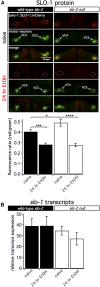
Symptoms of withdrawal from chronic alcohol use are a driving force for relapse in alcohol dependence. Thus, uncovering molecular targets to lessen their severity is key to breaking the cycle of dependence. Using the nematode Caenorhabditis elegans, we tested whether one highly conserved ethanol target, the large-conductance, calcium-activated potassium channel (known as the BK channel or Slo1), modulates ethanol withdrawal. Consistent with a previous report, we found that C. elegans displays withdrawal-related behavioral impairments after cessation of chronic ethanol exposure. We found that the degree of impairment is exacerbated in worms lacking the worm BK channel, SLO-1, and is reduced by selective rescue of this channel in the nervous system. Enhanced SLO-1 function, via gain-of-function mutation or overexpression, also dramatically reduced behavioral impairment during withdrawal. Consistent with these results, we found that chronic ethanol exposure decreased SLO-1 expression in a subset of neurons. In addition, we found that the function of a distinct, conserved Slo family channel, SLO-2, showed an inverse relationship to withdrawal behavior, and this influence depended on SLO-1 function. Together, our findings show that modulation of either Slo family ion channel bidirectionally regulates withdrawal behaviors in worm, supporting further exploration of the Slo family as targets for normalizing behaviors during alcohol withdrawal.
Keywords: alcohol; behavior; ethanol; potassium channel; slo-1; withdrawal.
Copyright © 2017 by the Genetics Society of America.
Figures



Similar articles
-
Putative calcium-binding domains of the Caenorhabditis elegans BK channel are dispensable for intoxication and ethanol activation.Genes Brain Behav. 2015 Jul;14(6):454-65. doi: 10.1111/gbb.12229. Genes Brain Behav. 2015. PMID: 26113050 Free PMC article.
-
Ethanol alters mechanosensory habituation in C. elegans by way of the BK potassium channel through a novel mechanism.PLoS One. 2025 Jun 11;20(6):e0315069. doi: 10.1371/journal.pone.0315069. eCollection 2025. PLoS One. 2025. PMID: 40498794 Free PMC article.
-
Conserved single residue in the BK potassium channel required for activation by alcohol and intoxication in C. elegans.J Neurosci. 2014 Jul 16;34(29):9562-73. doi: 10.1523/JNEUROSCI.0838-14.2014. J Neurosci. 2014. PMID: 25031399 Free PMC article.
-
Worms take to the slo lane: a perspective on the mode of action of emodepside.Invert Neurosci. 2012 Jun;12(1):29-36. doi: 10.1007/s10158-012-0133-x. Epub 2012 Apr 27. Invert Neurosci. 2012. PMID: 22539031 Free PMC article. Review.
-
Ethanol interactions with calcium-dependent potassium channels.Alcohol Clin Exp Res. 2007 Oct;31(10):1625-32. doi: 10.1111/j.1530-0277.2007.00469.x. Alcohol Clin Exp Res. 2007. PMID: 17850640 Review.
Cited by
-
Transcriptional analysis of the response of C. elegans to ethanol exposure.Sci Rep. 2021 May 26;11(1):10993. doi: 10.1038/s41598-021-90282-8. Sci Rep. 2021. PMID: 34040055 Free PMC article.
-
Toxicokinetics of the Antidepressant Fluoxetine and Its Active Metabolite Norfluoxetine in Caenorhabditis elegans and Their Comparative Potency.Environ Sci Technol. 2024 Feb 11;58(7):3129-40. doi: 10.1021/acs.est.3c07744. Online ahead of print. Environ Sci Technol. 2024. PMID: 38343161 Free PMC article.
-
Mitochondria in the Spotlight: C. elegans as a Model Organism to Evaluate Xenobiotic-Induced Dysfunction.Cells. 2023 Aug 22;12(17):2124. doi: 10.3390/cells12172124. Cells. 2023. PMID: 37681856 Free PMC article. Review.
-
Alcohol induces mitochondrial fragmentation and stress responses to maintain normal muscle function in Caenorhabditis elegans.FASEB J. 2020 Jun;34(6):8204-8216. doi: 10.1096/fj.201903166R. Epub 2020 Apr 15. FASEB J. 2020. PMID: 32294300 Free PMC article.
-
Calcium levels in ASER neurons determine behavioral valence by engaging distinct neuronal circuits in C. elegans.Nat Commun. 2025 Feb 20;16(1):1814. doi: 10.1038/s41467-025-57051-x. Nat Commun. 2025. PMID: 39979341 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

