Myeloid-derived suppressor cells-a new therapeutic target to overcome resistance to cancer immunotherapy
- PMID: 28546500
- PMCID: PMC6608049
- DOI: 10.1189/jlb.5VMR1116-458RRR
Myeloid-derived suppressor cells-a new therapeutic target to overcome resistance to cancer immunotherapy
Abstract
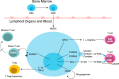
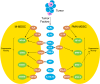
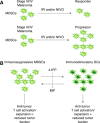
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells that accumulate during pathologic conditions, such as cancer. Patients diagnosed with advanced metastatic cancers have an average survival of 12-24 mo, a survival time that hasn't changed significantly in the past 30 yr. Despite some encouraging improvements in response rates and overall survival in patients receiving immunotherapies, such as immune checkpoint inhibitors, most patients will ultimately progress. MDSCs contribute to immunotherapeutic resistance by actively inhibiting antitumor T cell proliferation and cytotoxic activity as well as by promoting expansion of protumorigenic T regulatory cells, thereby, dampening the host immune responses against the tumor. In addition, MDSCs promote angiogenesis, tumor invasion, and metastasis. Thus, MDSCs are potential therapeutic targets in cases of multiple cancers. This review focuses on the phenotypic and functional characteristics of MDSCs and provides an overview of the mono- and combinatorial-therapeutic strategies that target MDSCs with an objective of enhancing the efficacy of cancer immunotherapies.
Keywords: MDSC; MIF; PFKFB3; immune checkpoint inhibitors; immune suppression; tumor immunology.
© Society for Leukocyte Biology.
Figures
References
-
- Hodi, F. S. , O'Day, S. J. , McDermott, D. F. , Weber, R. W. , Sosman, J. A. , Haanen, J. B. , Gonzalez, R. , Robert, C. , Schadendorf, D. , Hassel, J. C. , Akerley, W. , van den Eertwegh, A. J. , Lutzky, J. , Lorigan, P. , Vaubel, J. M. , Linette, G. P. , Hogg, D. , Ottensmeier, C. H. , Lebbé, C. , Peschel, C. , Quirt, I. , Clark, J. I. , Wolchok, J. D. , Weber, J. S. , Tian, J. , Yellin, M. J. , Nichol, G. M. , Hoos, A. , Urba, W. J. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723. - PMC - PubMed
-
- Robert, C. , Long, G. V. , Brady, B. , Dutriaux, C. , Maio, M. , Mortier, L. , Hassel, J. C. , Rutkowski, P. , McNeil, C. , Kalinka‐Warzocha, E. , Savage, K. J. , Hernberg, M. M. , Lebbé, C. , Charles, J. , Mihalcioiu, C. , Chiarion‐Sileni, V. , Mauch, C. , Cognetti, F. , Arance, A. , Schmidt, H. , Schadendorf, D. , Gogas, H. , Lundgren‐Eriksson, L. , Horak, C. , Sharkey, B. , Waxman, I. M. , Atkinson, V. , Ascierto, P. A. (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330. - PubMed
-
- Postow, M. A. , Chesney, J. , Pavlick, A. C. , Robert, C. , Grossmann, K. , McDermott, D. , Linette, G. P. , Meyer, N. , Giguere, J. K. , Agarwala, S. S. , Shaheen, M. , Ernstoff, M. S. , Minor, D. , Salama, A. K. , Taylor, M. , Ott, P. A. , Rollin, L. M. , Horak, C. , Gagnier, P. , Wolchok, J. D. , Hodi, F. S. (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017. - PMC - PubMed
-
- Wolchok, J. D. , Saenger, Y. (2008) The mechanism of anti‐CTLA‐4 activity and the negative regulation of T‐cell activation. Oncologist 13 (Suppl 4), 2–9. - PubMed
-
- Dranoff, G. (2009) Targets of protective tumor immunity. Ann. N. Y. Acad. Sci. 1174, 74–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

