A sensitive mass spectrometry platform identifies metabolic changes of life history traits in C. elegans
- PMID: 28546536
- PMCID: PMC5445081
- DOI: 10.1038/s41598-017-02539-w
A sensitive mass spectrometry platform identifies metabolic changes of life history traits in C. elegans
Abstract
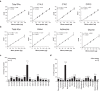
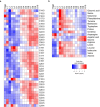
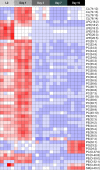
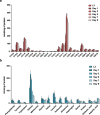
Abnormal nutrient metabolism is a hallmark of aging, and the underlying genetic and nutritional framework is rapidly being uncovered, particularly using C. elegans as a model. However, the direct metabolic consequences of perturbations in life history of C. elegans remain to be clarified. Based on recent advances in the metabolomics field, we optimized and validated a sensitive mass spectrometry (MS) platform for identification of major metabolite classes in worms and applied it to study age and diet related changes. Using this platform that allowed detection of over 600 metabolites in a sample of 2500 worms, we observed marked changes in fatty acids, amino acids and phospholipids during worm life history, which were independent from the germ-line. Worms underwent a striking shift in lipid metabolism after early adulthood that was at least partly controlled by the metabolic regulator AAK-2/AMPK. Most amino acids peaked during development, except aspartic acid and glycine, which accumulated in aged worms. Dietary intervention also influenced worm metabolite profiles and the regulation was highly specific depending on the metabolite class. Altogether, these MS-based methods are powerful tools to perform worm metabolomics for aging and metabolism-oriented studies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

