Emerging treatment options for the management of Hodgkin's lymphoma: clinical utility of nivolumab
- PMID: 28546779
- PMCID: PMC5436782
- DOI: 10.2147/JBM.S117452
Emerging treatment options for the management of Hodgkin's lymphoma: clinical utility of nivolumab
Abstract
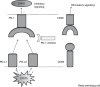
Classical Hodgkin's lymphoma (cHL) is a B-cell malignancy comprised of pathologic Reed Sternberg cells with a surrounding immune-tolerant inflammatory milieu. RS cells evade immune recognition in part through programmed death ligand 1 (PD-L1) overexpression, which is genetically programmed through copy number alterations, polysomy, and amplification of the 9p24.1 locus encoding PD-L1. By engaging with PD-1+ T-cells, PD-L1 delivers a potent immune suppressive signal promoting immunologic escape of the tumor cell. Enhancing antitumor immune response by targeting PD-1 with the monoclonal antibody nivolumab has proved to be effective in multiple solid tumors, but the highest response rates to date have been reported in patients with cHL, with over 65% of treated patients achieving an objective clinical response. In this review, we will summarize the published evidence regarding the activity of nivolumab in cHL as well as its current place in therapy. We will review the pharmacology, mechanism of action, and side effects of nivolumab as well as the emerging data indicating possible increased risk of graft versus host disease in patients treated with PD-1 inhibitors either pre- or post-allogeneic stem cell transplant. Given the remarkable single-agent activity and safety profile of PD-1 inhibitors in heavily pretreated patients with cHL, the possibility of employing nivolumab in combination with other active agents and earlier in therapy is a promising area of active investigation, and we will briefly summarize current clinical trials.
Keywords: Hodgkin’s lymphoma; checkpoint inhibitor therapy; nivolumab; pembrolizumab.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Ansell SM. Hodgkin lymphoma: MOPP chemotherapy to PD-1 blockade and beyond. Am J Hematol. 2016;91(1):109–112. - PubMed
-
- Armitage JO. Early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):653–662. - PubMed
-
- Johnson PW, Radford JA, Cullen MH, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma: results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519) J Clin Oncol. 2005;23(36):9208–9218. - PubMed
-
- Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27(32):5390–5396. - PubMed
-
- Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31(6):684–691. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

