Efficient isolation of Magnolia protoplasts and the application to subcellular localization of MdeHSF1
- PMID: 28546825
- PMCID: PMC5442663
- DOI: 10.1186/s13007-017-0193-3
Efficient isolation of Magnolia protoplasts and the application to subcellular localization of MdeHSF1
Abstract
Background: Magnolia is a woody ornamental plant, which is widely used in urban landscaping. However, its lengthy juvenile period and recalcitrance to regeneration impedes functional characterization of its genes.
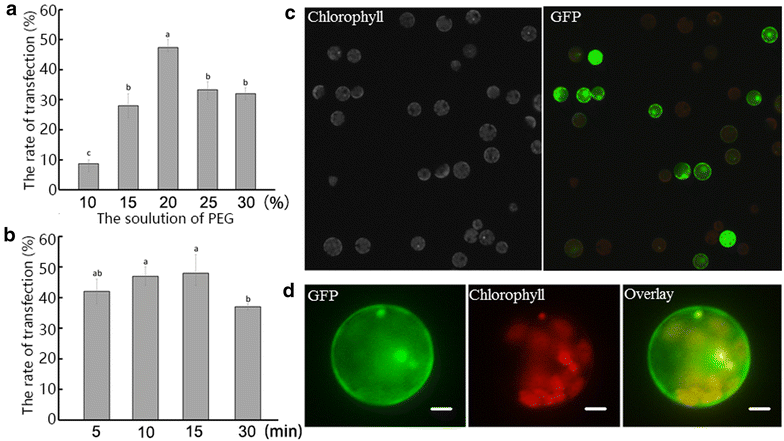
Results: We developed an efficient protoplast isolation and transient expression system for Magnolia denudata × Magnolia acuminata 'Yellow River'. The highest yield of protoplasts was obtained from young leaves digested in 3% Cellulase R10, 0.8% Macerozyme R10, 0.04% pectinase and 0.4 M mannitol enzymolysis solution for 6 h. For transfection of protoplasts, 20% PEG4000 for 5 min was optimal. To verify the protoplast system and begin to understand heat tolerance in Magnolia, a heat shock transcription factor MdeHSF1 was cloned from 'Yellow River', which belongs to the HSF subfamily A and has significant homology with AtHSFA1A. Subcellular localization analysis indicated that MdeHSF1 was expressed in the cell nucleus. Furthermore, qPCR analysis of the MdeHSF1 transcript level in response to high temperature stress suggested that MdeHSF1 might be involved in regulating heat stress tolerance in 'Yellow River'.
Conclusion: The described protocol provides a simple and straightforward method for isolating protoplast and exploring gene subcellular localization of MdeHSF1 in Magnolia. This expands the new research of protoplast isolation and transfection in Magnolia.
Keywords: Heat shock transcription factor; Heat stress; Magnolia; Protoplast isolation; Subcellular localization.
Figures






References
-
- Law Y, Xia N, Yang H. The origin, evolution and phytogeography of Magnoliaceae. J Trop Subtrop Bot. 1995;3(4):1–12.
LinkOut - more resources
Full Text Sources
Other Literature Sources

