Improving the thermostability of a fungal GH11 xylanase via site-directed mutagenesis guided by sequence and structural analysis
- PMID: 28546828
- PMCID: PMC5442702
- DOI: 10.1186/s13068-017-0824-y
Improving the thermostability of a fungal GH11 xylanase via site-directed mutagenesis guided by sequence and structural analysis
Abstract
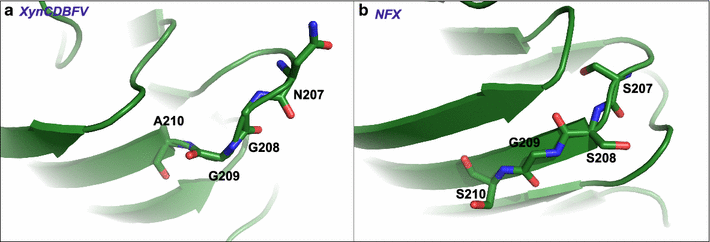
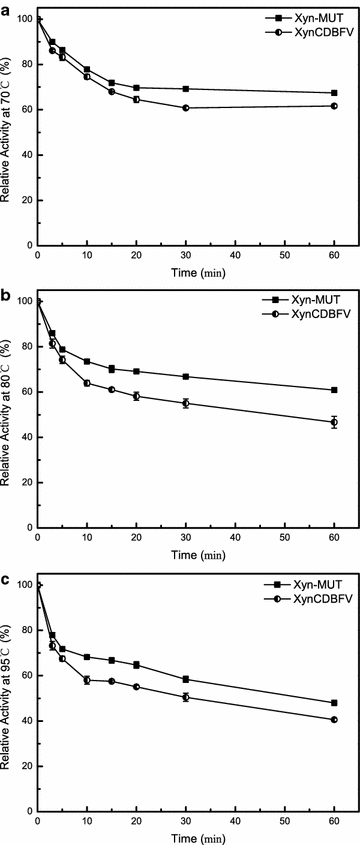
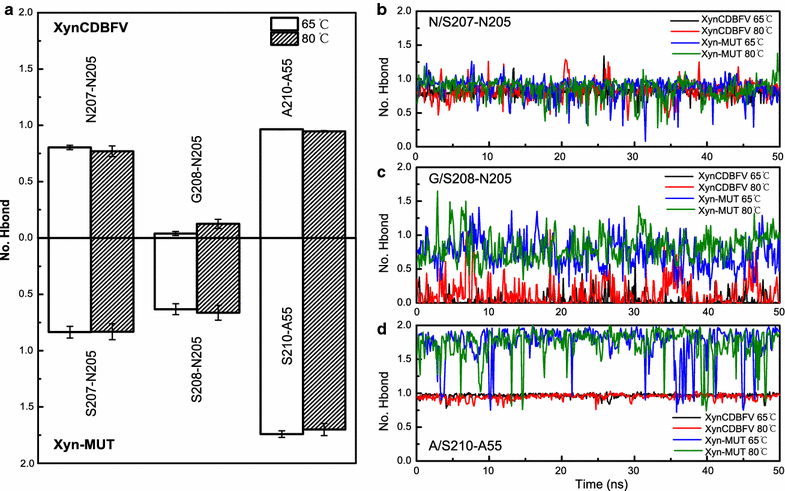
Background: Xylanases have been widely employed in many industrial processes, and thermophilic xylanases are in great demand for meeting the high-temperature requirements of biotechnological treatments. In this work, we aim to improve the thermostability of XynCDBFV, a glycoside hydrolase (GH) family 11 xylanase from the ruminal fungus Neocallimastix patriciarum, by site-directed mutagenesis. We report favorable mutations at the C-terminus from B-factor comparison and multiple sequence alignment.
Results: C-terminal residues 207-NGGA-210 in XynCDBFV were discovered to exhibit pronounced flexibility based on comparison of normalized B-factors. Multiple sequence alignment revealed that beneficial residues 207-SSGS-210 are highly conserved in GH11 xylanases. Thus, a recombinant xylanase, Xyn-MUT, was constructed by substituting three residues (N207S, G208S, A210S) at the C-terminus of XynCDBFV. Xyn-MUT exhibited higher thermostability than XynCDBFV at ≥70 °C. Xyn-MUT showed promising improvement in residual activity with a thermal retention of 14% compared to that of XynCDBFV after 1 h incubation at 80 °C; Xyn-MUT maintained around 50% of the maximal activity after incubation at 95 °C for 1 h. Kinetic measurements showed that the recombinant Xyn-MUT had greater kinetic efficiency than XynCDBFV (Km, 0.22 and 0.59 µM, respectively). Catalytic efficiency values (kcat/Km) of Xyn-MUT also increased (1.64-fold) compared to that of XynCDBFV. Molecular dynamics simulations were performed to explore the improved catalytic efficiency and thermostability: (1) the substrate-binding cleft of Xyn-MUT prefers to open to a larger extent to allow substrate access to the active site residues, and (2) hydrogen bond pairs S208-N205 and S210-A55 in Xyn-MUT contribute significantly to the improved thermostability. In addition, three xylanases with single point mutations were tested, and temperature assays verified that the substituted residues S208 and S210 give rise to the improved thermostability.
Conclusions: This is the first report for GH11 recombinant with improved thermostability based on C-terminus replacement. The resulting Xyn-MUT will be an attractive candidate for industrial applications.
Keywords: B-factor; C-terminus replacement; MD simulation; Site-directed mutagenesis; Thermostability; Xylanase.
Figures







Similar articles
-
Enhancing thermal tolerance of a fungal GH11 xylanase guided by B-factor analysis and multiple sequence alignment.Enzyme Microb Technol. 2019 Dec;131:109422. doi: 10.1016/j.enzmictec.2019.109422. Epub 2019 Sep 4. Enzyme Microb Technol. 2019. PMID: 31615659
-
Structural analysis of a glycoside hydrolase family 11 xylanase from Neocallimastix patriciarum: insights into the molecular basis of a thermophilic enzyme.J Biol Chem. 2014 Apr 18;289(16):11020-11028. doi: 10.1074/jbc.M114.550905. Epub 2014 Mar 11. J Biol Chem. 2014. PMID: 24619408 Free PMC article.
-
Impact of an N-terminal extension on the stability and activity of the GH11 xylanase from Thermobacillus xylanilyticus.J Biotechnol. 2014 Mar 20;174:64-72. doi: 10.1016/j.jbiotec.2014.01.004. Epub 2014 Jan 15. J Biotechnol. 2014. PMID: 24440633
-
Improving the catalytic performance of a GH11 xylanase by rational protein engineering.Appl Microbiol Biotechnol. 2015 Nov;99(22):9503-10. doi: 10.1007/s00253-015-6712-0. Epub 2015 Jun 19. Appl Microbiol Biotechnol. 2015. PMID: 26088174
-
Bacterial xylanases: biology to biotechnology.3 Biotech. 2016 Dec;6(2):150. doi: 10.1007/s13205-016-0457-z. Epub 2016 Jun 30. 3 Biotech. 2016. PMID: 28330222 Free PMC article. Review.
Cited by
-
Extremophilic Prokaryotic Endoxylanases: Diversity, Applicability, and Molecular Insights.Front Microbiol. 2021 Sep 9;12:728475. doi: 10.3389/fmicb.2021.728475. eCollection 2021. Front Microbiol. 2021. PMID: 34566933 Free PMC article. Review.
-
PEGASUS: Prediction of MD-derived protein flexibility from sequence.Protein Sci. 2025 Aug;34(8):e70221. doi: 10.1002/pro.70221. Protein Sci. 2025. PMID: 40671366 Free PMC article.
-
Carbohydrate active enzyme domains from extreme thermophiles: components of a modular toolbox for lignocellulose degradation.Extremophiles. 2018 Jan;22(1):1-12. doi: 10.1007/s00792-017-0974-7. Epub 2017 Nov 6. Extremophiles. 2018. PMID: 29110088 Review.
-
Improving the Thermostability of Rhizopus chinensis Lipase Through Site-Directed Mutagenesis Based on B-Factor Analysis.Front Microbiol. 2020 Mar 3;11:346. doi: 10.3389/fmicb.2020.00346. eCollection 2020. Front Microbiol. 2020. PMID: 32194535 Free PMC article.
-
Exploring Bacillus species xylanases for industrial applications: screening via thermostability and reaction modelling.J Mol Model. 2024 Jul 2;30(8):242. doi: 10.1007/s00894-024-06048-2. J Mol Model. 2024. PMID: 38955857
References
-
- Taibi Z, Saoudi B, Boudelaa M, Trigui H, Belghith H, Gargouri A, Ladjama A. Purification and biochemical characterization of a highly thermostable xylanase from Actinomadura sp strain Cpt20 isolated from poultry compost. Appl Biochem Biotech. 2012;166:663–679. doi: 10.1007/s12010-011-9457-y. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

