A practical and efficient approach to imidazo[1,2- a]pyridine-fused isoquinolines through the post-GBB transformation strategy
- PMID: 28546839
- PMCID: PMC5433183
- DOI: 10.3762/bjoc.13.82
A practical and efficient approach to imidazo[1,2- a]pyridine-fused isoquinolines through the post-GBB transformation strategy
Abstract
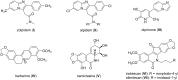
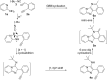
Diversity-oriented synthesis of the biologically intriguing imidazo[1,2-a]pyridine-fused isoquinoline systems from readily available starting materials was achieved through the Groebke-Blackburn-Bienaymé reaction followed by a gold-catalyzed cyclization strategy. The synthetic approach is characterized by mild reaction conditions and a broad substrate scope, allowing for the rapid construction of structurally complex and diverse heterocycles in moderate to good yields.
Keywords: Groebke–Blackburn–Bienaymé reaction; Ugi reaction; imidazo[1,2-a]pyridines; isoquinolines; multicomponent reaction.
Figures
Similar articles
-
Diversity-Oriented Synthesis of [2.2]Paracyclophane-derived Fused Imidazo[1,2-a]heterocycles by Groebke-Blackburn-Bienaymé Reaction: Accessing Cyclophanyl Imidazole Ligands Library.Chemistry. 2022 Jan 13;28(3):e202103511. doi: 10.1002/chem.202103511. Epub 2021 Dec 8. Chemistry. 2022. PMID: 34792822 Free PMC article.
-
Synthesis of imidazo[1,2-a]pyridine-containing peptidomimetics by tandem of Groebke-Blackburn-Bienaymé and Ugi reactions.Beilstein J Org Chem. 2023 May 26;19:727-735. doi: 10.3762/bjoc.19.53. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37284590 Free PMC article.
-
Synthesis of Imidazo[1,2-a]pyridine-Fused 1,3-Benzodiazepine Derivatives with Anticancer Activity via a One-Pot Cascade GBB-3CR/Pd(II)-Catalyzed Azide-Isocyanide Coupling/Cyclization Process.J Org Chem. 2023 Sep 15;88(18):13125-13134. doi: 10.1021/acs.joc.3c01341. Epub 2023 Aug 24. J Org Chem. 2023. PMID: 37616489
-
The Groebke-Blackburn-Bienaymé reaction in its maturity: innovation and improvements since its 21st birthday (2019-2023).Beilstein J Org Chem. 2024 Aug 1;20:1839-1879. doi: 10.3762/bjoc.20.162. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39109293 Free PMC article. Review.
-
Trends in the Synthesis of Antimicrobial Derivatives by using the Gewald, Strecker, and Groebke-Blackburn-Bienaymé (GBB) Reactions.Med Chem. 2024;20(7):663-688. doi: 10.2174/0115734064282699240315042428. Med Chem. 2024. PMID: 38523542 Review.
Cited by
-
The Groebke-Blackburn-Bienaymé Reaction.Eur J Chem. 2019 Nov 14;2019(42):7007-7049. doi: 10.1002/ejoc.201901124. Epub 2019 Aug 30. Eur J Chem. 2019. PMID: 34012704 Free PMC article.
-
Aminoazole-Based Diversity-Oriented Synthesis of Heterocycles.Front Chem. 2018 Nov 13;6:527. doi: 10.3389/fchem.2018.00527. eCollection 2018. Front Chem. 2018. PMID: 30555815 Free PMC article. Review.
-
Novel 5-Nitrofuran-Tagged Imidazo-Fused Azines and Azoles Amenable by the Groebke-Blackburn-Bienaymé Multicomponent Reaction: Activity Profile against ESKAPE Pathogens and Mycobacteria.Biomedicines. 2022 Sep 6;10(9):2203. doi: 10.3390/biomedicines10092203. Biomedicines. 2022. PMID: 36140307 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources