Recent Progress in Energy-Driven Water Splitting
- PMID: 28546906
- PMCID: PMC5441509
- DOI: 10.1002/advs.201600337
Recent Progress in Energy-Driven Water Splitting
Abstract
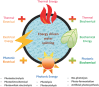
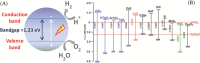
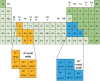
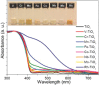
Hydrogen is readily obtained from renewable and non-renewable resources via water splitting by using thermal, electrical, photonic and biochemical energy. The major hydrogen production is generated from thermal energy through steam reforming/gasification of fossil fuel. As the commonly used non-renewable resources will be depleted in the long run, there is great demand to utilize renewable energy resources for hydrogen production. Most of the renewable resources may be used to produce electricity for driving water splitting while challenges remain to improve cost-effectiveness. As the most abundant energy resource, the direct conversion of solar energy to hydrogen is considered the most sustainable energy production method without causing pollutions to the environment. In overall, this review briefly summarizes thermolytic, electrolytic, photolytic and biolytic water splitting. It highlights photonic and electrical driven water splitting together with photovoltaic-integrated solar-driven water electrolysis.
Keywords: electrochemical water splitting; hydrogen generation; photocatalytic water splitting; photoelectrochemical water splitting; solar water splitting.
Figures



References
-
- Christopher K., Dimitrios R., Energy Environ. Sci. 2012, 5, 6640.
-
- Azwar M. Y., Hussain M. A., Abdul‐Wahab A. K., Renew. Sustainable Energy Rev. 2014, 31, 158.
-
- Steinfeld A., Sol. Energy 2005, 78, 603.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
