Challenges in the delivery of therapies to melanoma brain metastases
- PMID: 28546917
- PMCID: PMC5440090
- DOI: 10.1007/s40495-016-0072-z
Challenges in the delivery of therapies to melanoma brain metastases
Abstract
Brain metastases are a major cause of morbidity and mortality in patients with advanced melanoma. Recent approval of several molecularly-targeted agents and biologics has brought hope to patients with this previously untreatable disease. However, patients with symptomatic melanoma brain metastases have often been excluded from pivotal clinical trials. This may be in part attributed to the fact that several of the approved small molecule molecularly-targeted agents are substrates for active efflux at the blood-brain barrier, limiting their effective delivery to brain metastases. We believe that successful treatment of melanoma brain metastases will depend on the ability of these agents to traverse the blood-brain barrier and reach micrometastases that are often not clinically detectable. Moreover, overcoming the emergence of a unique pattern of resistance, possibly through adequate delivery of combination targeted therapies in brain metastases will be important in achieving a durable response. These concepts, and the current challenges in the delivery of new treatments to melanoma brain metastases, are discussed in this review.
Keywords: active efflux; blood-brain barrier; drug delivery; drug resistance; melanoma brain metastases; molecularly-targeted agents.
Conflict of interest statement
Conflict of Interest Statement: The authors indicate no conflict of interest with the subject matter of this review.
Figures
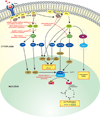





Similar articles
-
Drug delivery to melanoma brain metastases: Can current challenges lead to new opportunities?Pharmacol Res. 2017 Sep;123:10-25. doi: 10.1016/j.phrs.2017.06.008. Epub 2017 Jun 17. Pharmacol Res. 2017. PMID: 28634084 Free PMC article.
-
Systemic therapy for brain metastases.Handb Clin Neurol. 2018;149:137-153. doi: 10.1016/B978-0-12-811161-1.00011-6. Handb Clin Neurol. 2018. PMID: 29307351 Review.
-
The use of systemic therapies for the treatment of brain metastases in metastatic melanoma: opportunities and unanswered questions.Cancer Treat Rev. 2013 Dec;39(8):833-8. doi: 10.1016/j.ctrv.2013.06.004. Epub 2013 Jul 9. Cancer Treat Rev. 2013. PMID: 23845462 Review.
-
Treatment of Melanoma CNS Metastases.Cancer Treat Res. 2016;167:263-79. doi: 10.1007/978-3-319-22539-5_11. Cancer Treat Res. 2016. PMID: 26601867 Review.
-
Targeted Treatment of Brain Metastases.Curr Neurol Neurosci Rep. 2017 Apr;17(4):37. doi: 10.1007/s11910-017-0741-2. Curr Neurol Neurosci Rep. 2017. PMID: 28326470 Review.
Cited by
-
Case report: A case of classic hairy cell leukemia with CNS involvement treated with vemurafenib.Front Oncol. 2023 Jan 12;12:1100577. doi: 10.3389/fonc.2022.1100577. eCollection 2022. Front Oncol. 2023. PMID: 36713531 Free PMC article.
-
Drug delivery to melanoma brain metastases: Can current challenges lead to new opportunities?Pharmacol Res. 2017 Sep;123:10-25. doi: 10.1016/j.phrs.2017.06.008. Epub 2017 Jun 17. Pharmacol Res. 2017. PMID: 28634084 Free PMC article.
-
Novel RAF-directed approaches to overcome current clinical limits and block the RAS/RAF node.Mol Oncol. 2024 Jun;18(6):1355-1377. doi: 10.1002/1878-0261.13605. Epub 2024 Feb 16. Mol Oncol. 2024. PMID: 38362705 Free PMC article. Review.
-
Leptomeningeal Metastases in Melanoma Patients: An Update on and Future Perspectives for Diagnosis and Treatment.Int J Mol Sci. 2023 Jul 14;24(14):11443. doi: 10.3390/ijms241411443. Int J Mol Sci. 2023. PMID: 37511202 Free PMC article. Review.
-
Stereotactic radiosurgery combined with targeted/ immunotherapy in patients with melanoma brain metastasis.Radiat Oncol. 2020 Feb 14;15(1):37. doi: 10.1186/s13014-020-1485-8. Radiat Oncol. 2020. PMID: 32059731 Free PMC article.
References
-
- Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36(3):437–449. - PubMed
-
- Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22(11):2101–2107. doi:10.1200/JCO.2004.11.044 JCO.2004.11.044 [pii]. - PubMed
-
- Ajithkumar T, Parkinson C, Fife K, Corrie P, Jefferies S. Evolving treatment options for melanoma brain metastases. Lancet Oncol. 2015;16(13):e486–e497. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
