Cell viability and extracellular matrix synthesis in a co-culture system of corneal stromal cells and adipose-derived mesenchymal stem cells
- PMID: 28546919
- PMCID: PMC5437450
- DOI: 10.18240/ijo.2017.05.02
Cell viability and extracellular matrix synthesis in a co-culture system of corneal stromal cells and adipose-derived mesenchymal stem cells
Abstract
Aim: To investigate the impact of adipose-derived mesenchymal stem cells (ADSCs) on cell viability and extracellular matrix (ECM) synthesis of corneal stromal cells (CSCs).
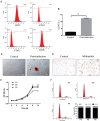
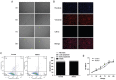
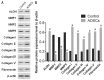
Methods: ADSCs and CSCs were obtained from the corneas of New Zealand white rabbits and indirectly co-cultured in vitro. The proliferative capacity of CSCs in the different groups was assessed by CCK-8 assays. Annexin V-fluorescein isothiocyanate (FITC)/proliferation indices (PI) assays were used to detect the apoptosis of CSCs. The expression levels of matrix metalloproteinase (MMP), such as MMP1, MMP2, MMP9, and collagens were also evaluated by Western blot.
Results: ADSCs significantly promoted proliferation and invasion of CSCs in the indirect co-culture assays. The co-cultural group displayed much higher ability of proliferation, especially under the co-culture conditions of ADSCs for 3d, compared with that CSCs cultured alone. The PI of CSCs in the co-culture system were increased approximately 3-8-fold compared with the control group. A significant change was observed in the proportions of cells at apoptosis (early and late) between the negative control group (6.34% and 2.06%) and the ADCSs-treated group (4.69% and 1.59%). The expression levels of MMPs were down regulated in the co-culture models. Compared with the control group, the decrease intensities of MMP-1, MMP-2 and MMP-9 in CSCs/ADSCs group were observed, 3.90-fold, 1.09-fold and 3.03-fold, respectively. However, the increase intensities of collagen type (I, II, III, IV, and V) in CSCs were observed in CSCs/ADSCs group, 3.47-fold, 4.30-fold, 2.35-fold, 2.55-fold and 2.43-fold, respectively, compared to that in the control group. The expressions of aldehyde dehydrogenase and fibronectin in CSCs were upregulated in the co-culture models.
Conclusion: ADSCs play a promotive role in CSCs' growth and invasion, which may be partially associated with MMPs decrease and collagens increase, resulting in a positive participation in the plasticity and ECM synthesis of CSCs. This provided a new insight into the extensive role of ADSCs in CSCs and a potential molecular target for corneal therapy.
Keywords: adipose-derived mesenchymal stem cell; corneal stromal cells; extracellular matrix; plasticity.
Figures
Similar articles
-
Effects of Adipose-derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis.Chin Med J (Engl). 2018 Mar 20;131(6):704-712. doi: 10.4103/0366-6999.226889. Chin Med J (Engl). 2018. PMID: 29521294 Free PMC article.
-
Adipose-derived mesenchymal stem cells promote cell proliferation and invasion of epithelial ovarian cancer.Exp Cell Res. 2015 Sep 10;337(1):16-27. doi: 10.1016/j.yexcr.2015.07.020. Epub 2015 Jul 21. Exp Cell Res. 2015. PMID: 26209607
-
Adipose-derived stromal cells protect intervertebral disc cells in compression: implications for stem cell regenerative disc therapy.Int J Biol Sci. 2015 Jan 1;11(2):133-43. doi: 10.7150/ijbs.10598. eCollection 2015. Int J Biol Sci. 2015. PMID: 25561896 Free PMC article.
-
Adipose Derived Stem Cells Affect miR-145 and p53 Expressions of Co-Cultured Hematopoietic Stem Cells.Cell J. 2018 Jan;19(4):654-659. doi: 10.22074/cellj.2018.4393. Epub 2017 Nov 4. Cell J. 2018. PMID: 29105402 Free PMC article.
-
Effect of adipose-derived mesenchymal stromal cells on tendon healing in aging and estrogen deficiency: an in vitro co-culture model.Cytotherapy. 2015 Nov;17(11):1536-44. doi: 10.1016/j.jcyt.2015.07.007. Epub 2015 Aug 21. Cytotherapy. 2015. PMID: 26305076
Cited by
-
Overexpression of klotho in adipose-derived stem cells protects against UVB-induced photoaging in co-cultured human fibroblasts.Mol Med Rep. 2018 Dec;18(6):5473-5480. doi: 10.3892/mmr.2018.9594. Epub 2018 Oct 25. Mol Med Rep. 2018. PMID: 30365106 Free PMC article.
-
Low-level laser and adipose-derived stem cells altered remodelling genes expression and improved collagen reorganization during tendon repair.Cell Prolif. 2019 May;52(3):e12580. doi: 10.1111/cpr.12580. Epub 2019 Feb 7. Cell Prolif. 2019. PMID: 30734394 Free PMC article.
-
Mesenchymal Stem Cells for Regenerative Medicine.Cells. 2019 Aug 13;8(8):886. doi: 10.3390/cells8080886. Cells. 2019. PMID: 31412678 Free PMC article. Review.
-
MicroRNA-124-3p affects myogenic differentiation of adipose-derived stem cells by targeting Caveolin-1 during pelvic floor dysfunction in Sprague Dawley rats.Ann Transl Med. 2021 Jan;9(2):161. doi: 10.21037/atm-20-8212. Ann Transl Med. 2021. PMID: 33569463 Free PMC article.
-
Effects of Adipose-derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis.Chin Med J (Engl). 2018 Mar 20;131(6):704-712. doi: 10.4103/0366-6999.226889. Chin Med J (Engl). 2018. PMID: 29521294 Free PMC article.
References
-
- Huang SJ, Fu RH, Shyu WC, Liu SP, Jong GP, Chiu YW, Wu HS, Tsou YA, Cheng CW, Lin SZ. Adipose-derived stem cells: isolation, characterization, and differentiation potential. Cell Transplant. 2013;22(4):701–709. - PubMed
-
- Marcus AJ, Coyne TM, Rauch J, Woodbury D, Black IB. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76(2):130–144. - PubMed
-
- Franco B, Vincenzo V, Alessandro DV, Tonello C, Abatangelo G. Bioengineered tendon: from tenocytes culture to ASCs (adipose derived stem cells) culture. Wound Repair Regen. 2008;16(6):A74.
-
- Vachkova E, Bosnakovski D, Yonkova P, Grigorova N, Ivanova Z, Todorov P, Penchev G, Milanova A, Simeonova G, Stanilova S, Georgiev IP. Adipogenic potential of stem cells derived from rabbit subcutaneous and visceral adipose tissue in vitro. In Vitro Cell Dev Biol Anim. 2016;52(8):829–837. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
