The cost and cost trajectory of whole-genome analysis guiding treatment of patients with advanced cancers
- PMID: 28546995
- PMCID: PMC5441418
- DOI: 10.1002/mgg3.281
The cost and cost trajectory of whole-genome analysis guiding treatment of patients with advanced cancers
Abstract
Background: Limited data exist on the real-world costs of applying whole-genome analysis (WGA) in a clinical setting. We estimated the costs of applying WGA to guide treatments for patients with advanced cancers and characterized how costs evolve over time.
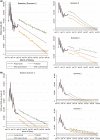
Methods: The setting is the British Columbia Cancer Agency Personalized OncoGenomics (POG) program in British Columbia, Canada. Cost data were obtained for patients who enrolled in the program from 2012 to 2015. We estimated mean WGA costs using bootstrapping. We applied time series analysis and produced 10-year forecasts to determine when costs are expected to reach critical thresholds.
Results: The mean cost of WGA over the study period was CDN$34,886 per patient (95% CI: $34,051, $35,721). Over time, WGA costs decreased, driven by a reduction in costs of sequencing. Yet, costs of other components of WGA increased. Forecasting showed WGA costs may not reach critical thresholds within the next 10 years.
Conclusion: WGA costs decreased over the studied time horizon, but expenditures needed to realize WGA remain significant. Future research exploring costs and benefits of WGA-guided cancer care are crucial to guide health policy.
Keywords: Cost analysis; oncology; transcriptome sequencing; whole‐genome sequencing.
Figures
References
-
- Barber, J. A. , and Thompson S. G.. 2000. Analysis of cost data in randomized trials: an application of the non‐parametric bootstrap. Stat. Med. 19:3219–3236. - PubMed
-
- Batley, J. , and Edwards D.. 2009. Genome sequence data: management, storage, and visualization. Biotechniques 46:333–334. - PubMed
-
- BC Cancer Statistics ‐ Facts and Figures . 2016. http://www.bccancer.bc.ca/health-info/disease-system-statistics/bc-cance.... (accessed 12 September 2016).
-
- CADTH . 2014. Next generation DNA sequencing: a review of the cost effectiveness and guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources