Perfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II-III diffuse gliomas
- PMID: 28547590
- PMCID: PMC7927357
- DOI: 10.1007/s11060-017-2506-9
Perfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II-III diffuse gliomas
Abstract
The value of perfusion and diffusion-weighted MRI in differentiating histological subtypes according to the 2007 WHO glioma classification scheme (i.e. astrocytoma vs. oligodendroglioma) and genetic subtypes according to the 2016 WHO reclassification (e.g. 1p/19q co-deletion and IDH1 mutation status) in WHO grade II and III diffuse gliomas remains controversial. In the current study, we describe unique perfusion and diffusion MR signatures between histological and genetic glioma subtypes. Sixty-five patients with 2007 histological designations (astrocytomas and oligodendrogliomas), 1p/19q status (+ = intact/- = co-deleted), and IDH1 mutation status (MUT/WT) were included in this study. In all patients, median relative cerebral blood volume (rCBV) and apparent diffusion coefficient (ADC) were estimated within T2 hyperintense lesions. Bootstrap hypothesis testing was used to compare subpopulations of gliomas, separated by WHO grade and 2007 or 2016 glioma classification schemes. A multivariable logistic regression model was also used to differentiate between 1p19q+ and 1p19q- WHO II-III gliomas. Neither rCBV nor ADC differed significantly between histological subtypes of pure astrocytomas and pure oligodendrogliomas. ADC was significantly different between molecular subtypes (p = 0.0016), particularly between IDHWT and IDHMUT/1p19q+ (p = 0.0013). IDHMUT/1p19q+ grade III gliomas had higher median ADC; IDHWT grade III gliomas had higher rCBV with lower ADC; and IDHMUT/1p19q- had intermediate rCBV and ADC values, similar to their grade II counterparts. A multivariable logistic regression model was able to differentiate between IDHWT and IDHMUT WHO II and III gliomas with an AUC of 0.84 (p < 0.0001, 74% sensitivity, 79% specificity). Within IDHMUT WHO II-III gliomas, a separate multivariable logistic regression model was able to differentiate between 1p19q+ and 1p19q- WHO II-III gliomas with an AUC of 0.80 (p = 0.0015, 64% sensitivity, 82% specificity). ADC better differentiated between genetic subtypes of gliomas according to the 2016 WHO guidelines compared to the classification scheme outlined in the 2007 WHO guidelines based on histological features of the tissue. Results suggest a combination of rCBV, ADC, T2 hyperintense volume, and presence of contrast enhancement together may aid in non-invasively identifying genetic subtypes of diffuse gliomas.
Keywords: Diffusion MRI; Glioma; IDH mutant; Perfusion MRI; WHO classification.
Figures
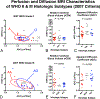




Similar articles
-
A Comparative Study of 2 Different Segmentation Methods of ADC Histogram for Differentiation Genetic Subtypes in Lower-Grade Diffuse Gliomas.Biomed Res Int. 2020 Sep 28;2020:9549361. doi: 10.1155/2020/9549361. eCollection 2020. Biomed Res Int. 2020. PMID: 33062706 Free PMC article.
-
Advanced imaging parameters improve the prediction of diffuse lower-grade gliomas subtype, IDH mutant with no 1p19q codeletion: added value to the T2/FLAIR mismatch sign.Eur Radiol. 2020 Feb;30(2):844-854. doi: 10.1007/s00330-019-06395-2. Epub 2019 Aug 24. Eur Radiol. 2020. PMID: 31446467
-
[Apparent Diffusion Coefficient Histogram Analysis:Differentiation of Genetic Subtypes of Diffuse Lower-grade Gliomas].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2020 Aug 30;42(4):444-451. doi: 10.3881/j.issn.1000-503X.11608. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2020. PMID: 32895095 Chinese.
-
The T2-FLAIR-mismatch sign as an imaging biomarker for IDH and 1p/19q status in diffuse low-grade gliomas: a systematic review with a Bayesian approach to evaluation of diagnostic test performance.Neurosurg Focus. 2019 Dec 1;47(6):E13. doi: 10.3171/2019.9.FOCUS19660. Neurosurg Focus. 2019. PMID: 31786548
-
Histomolecular classification of adult diffuse gliomas: the diagnostic value of immunohistochemical markers.Rev Neurol (Paris). 2011 Oct;167(10):683-90. doi: 10.1016/j.neurol.2011.07.006. Epub 2011 Sep 1. Rev Neurol (Paris). 2011. PMID: 21889777 Review.
Cited by
-
A Comparative Study of 2 Different Segmentation Methods of ADC Histogram for Differentiation Genetic Subtypes in Lower-Grade Diffuse Gliomas.Biomed Res Int. 2020 Sep 28;2020:9549361. doi: 10.1155/2020/9549361. eCollection 2020. Biomed Res Int. 2020. PMID: 33062706 Free PMC article.
-
Targeting Isocitrate Dehydrogenase (IDH) in Solid Tumors: Current Evidence and Future Perspectives.Cancers (Basel). 2024 Aug 2;16(15):2752. doi: 10.3390/cancers16152752. Cancers (Basel). 2024. PMID: 39123479 Free PMC article. Review.
-
Clinicopathological analysis of T2-FLAIR mismatch sign in lower-grade gliomas.Sci Rep. 2020 Jun 22;10(1):10113. doi: 10.1038/s41598-020-67244-7. Sci Rep. 2020. PMID: 32572107 Free PMC article.
-
Molecular Subtype Classification in Lower-Grade Glioma with Accelerated DTI.AJNR Am J Neuroradiol. 2019 Sep;40(9):1458-1463. doi: 10.3174/ajnr.A6162. Epub 2019 Aug 14. AJNR Am J Neuroradiol. 2019. PMID: 31413006 Free PMC article.
-
Visualization of tumor heterogeneity and prediction of isocitrate dehydrogenase mutation status for human gliomas using multiparametric physiologic and metabolic MRI.Sci Rep. 2022 Jan 20;12(1):1078. doi: 10.1038/s41598-022-05077-2. Sci Rep. 2022. PMID: 35058510 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

