Lentivirus-Induced Dendritic Cells (iDC) for Immune-Regenerative Therapies in Cancer and Stem Cell Transplantation
- PMID: 28548069
- PMCID: PMC5344221
- DOI: 10.3390/biomedicines2030229
Lentivirus-Induced Dendritic Cells (iDC) for Immune-Regenerative Therapies in Cancer and Stem Cell Transplantation
Abstract
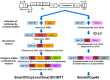
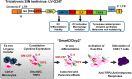
Conventional dendritic cells (cDC) are ex vivo differentiated professional antigen presenting cells capable of potently stimulating naïve T cells and with vast potential for immunotherapeutic applications. The manufacture of clinical-grade cDC is relatively complex and requires several days for completion. Clinical trials showed poor trafficking of cDC from subcutaneous injection sites to lymph nodes (LN), where DC can optimally stimulate naïve lymphocytes for long-lasting memory responses. We demonstrated in mouse and human systems that a single overnight ex vivo lentiviral (LV) gene transfer into DC precursors for production of combination of cytokines and antigens was capable to induce autonomous self-differentiation of antigen-loaded DC in vitro and in vivo. These highly viable induced DC (iDC) effectively migrated from the injected skin to LN, where they effectively activated de novo antigen-specific effector memory T cells. Two iDC modalities were validated in relevant animal models and are now in clinical development: Self-differentiated Myeloid-derived Antigen-presenting-cells Reactive against Tumors co-expressing GM-CSF/IL-4/TRP2 for melanoma immunotherapy in the autologous setting (SmartDCtrp2), and Self-differentiated Myeloid-derived Lentivirus-induced against human cytomegalovirus as an allogeneic matched adoptive cell after stem cell transplantation (SmyleDCpp65). The lentiviral vector design and packaging methodology has "evolved" continuously in order to simplify and optimize function and biosafety of in vitro and in vivo genetic reprogramming of iDC. Here, we address the challenges seeking for new creations of genetically programmed iDC and integrase-defective LV vaccines for immune regeneration.
Keywords: cancer; dendritic cells; lentiviral vectors; stem cell transplantation.
Conflict of interest statement
A patent application describing the invention of iDC for use in stem cell transplantation is pending.
Figures

References
-
- Schadendorf D., Ugurel S., Schuler-Thurner B., Nestle F.O., Enk A., Brocker E.B., Grabbe S., Rittgen W., Edler L., Sucker A., et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: A randomized phase III trial of the DC study group of the DeCOG. Ann. Oncol. 2006;17:563–570. doi: 10.1093/annonc/mdj138. - DOI - PubMed
-
- Verdijk P., Aarntzen E.H., Lesterhuis W.J., Boullart A.C., Kok E., van Rossum M.M., Strijk S., Eijckeler F., Bonenkamp J.J., Jacobs J.F., et al. Limited amounts of dendritic cells migrate into the T-cell area of lymph nodes but have high immune activating potential in melanoma patients. Clin. Cancer Res. 2009;15:2531–2540. doi: 10.1158/1078-0432.CCR-08-2729. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

