Fast computation of full density matrix of multispin systems for spatially localized in vivo magnetic resonance spectroscopy
- PMID: 28548302
- PMCID: PMC5578626
- DOI: 10.1002/mp.12375
Fast computation of full density matrix of multispin systems for spatially localized in vivo magnetic resonance spectroscopy
Abstract
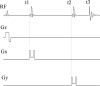
Purpose: Numerical simulations of three-dimensionally localized MRS spectra have been very time consuming for multispin systems because the current state-of-the-art method requires computation of a large ensemble of spins pixel-by-pixel in three dimensional space. This paper describes a highly accelerated technique for computing spatially localized MRS spectra using the full solution to the Liouville-von Neumann equation.
Methods: The time evolution of spatially localized multispin density matrix as the full solution to the Liouville-von Neumann equation was analyzed. A new technique based on one dimensional spatial projection of the full density matrix was proposed. This method was implemented using a computer program written in Java language.
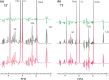
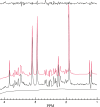
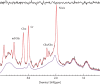
Results: The MRS spectra calculated using the new method were found to be identical to conventional three-dimensional simulation for the same digitization of the voxel while the new method reduced computation time by orders of magnitude and led to not only improved speed but also accuracy. Applications of the new method to phantom studies of multispin systems and quantification of in vivo MRS spectra of brain were demonstrated.
Conclusion: The dramatically enhanced computational efficiency makes accurate simulation of localized MRS spectra highly accessible for calculating basis sets for spectral quantification and for optimizing pulse sequences.
Keywords: MRS quantification; localized MRS; multispin density operator; numerical simulation; one-dimensional projection; shaped RF pulse.
Published 2017. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
The authors report no conflict of interest and have no financial disclosures.
Figures



References
-
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. - PubMed
-
- Smith SA, Levante TO, Meier BH, Ernst RR. Computer simulations in magnetic resonance. An object‐oriented programming approach. J Magn Reson A. 1994;106:75–105.
-
- Hancu I, Port J. The case of the missing glutamine. NMR Biomed. 2011;24:529–535. - PubMed
-
- Hur R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE‐averaged PRESS at 3 T. Magn Reson Med. 2004;51:435–440. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

