EMT and MET: necessary or permissive for metastasis?
- PMID: 28548345
- PMCID: PMC5496498
- DOI: 10.1002/1878-0261.12083
EMT and MET: necessary or permissive for metastasis?
Abstract
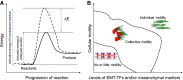
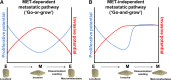
Epithelial-to-mesenchymal transition (EMT) and its reverse mesenchymal-to-epithelial transition (MET) have been suggested to play crucial roles in metastatic dissemination of carcinomas. These phenotypic transitions between states are not binary. Instead, carcinoma cells often exhibit a spectrum of epithelial/mesenchymal phenotype(s). While epithelial/mesenchymal plasticity has been observed preclinically and clinically, whether any of these phenotypic transitions are indispensable for metastatic outgrowth remains an unanswered question. Here, we focus on epithelial/mesenchymal plasticity in metastatic dissemination and propose alternative mechanisms for successful dissemination and metastases beyond the traditional EMT/MET view. We highlight multiple hypotheses that can help reconcile conflicting observations, and outline the next set of key questions that can offer valuable insights into mechanisms of metastasis in multiple tumor models.
Keywords: epithelial-to-mesenchymal transition; hybrid epithelial/mesenchymal; mesenchymal-to-epithelial transition; metastasis; phenotypic plasticity.
© 2017 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures

References
-
- Andriani F, Bertolini G, Facchinetti F, Baldoli E, Moro M, Casalini P, Caserini R, Milione M, Leone G, Pelosi G et al (2016) Conversion to stem‐cell state in response to microenvironmental cues is regulated by balance between epithelial and mesenchymal features in lung cancer cells. Mol Oncol 10, 253–271. - PMC - PubMed
-
- Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ and Garcia‐Blanco MA (2011) Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res 9, 997–1007. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

