Rai1 frees mice from the repression of active wake behaviors by light
- PMID: 28548639
- PMCID: PMC5464769
- DOI: 10.7554/eLife.23292
Rai1 frees mice from the repression of active wake behaviors by light
Abstract
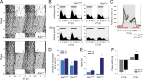
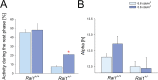
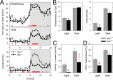
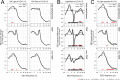
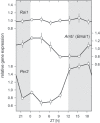
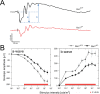
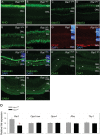
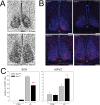
Besides its role in vision, light impacts physiology and behavior through circadian and direct (aka 'masking') mechanisms. In Smith-Magenis syndrome (SMS), the dysregulation of both sleep-wake behavior and melatonin production strongly suggests impaired non-visual light perception. We discovered that mice haploinsufficient for the SMS causal gene, Retinoic acid induced-1 (Rai1), were hypersensitive to light such that light eliminated alert and active-wake behaviors, while leaving time-spent-awake unaffected. Moreover, variables pertaining to circadian rhythm entrainment were activated more strongly by light. At the input level, the activation of rod/cone and suprachiasmatic nuclei (SCN) by light was paradoxically greatly reduced, while the downstream activation of the ventral-subparaventricular zone (vSPVZ) was increased. The vSPVZ integrates retinal and SCN input and, when activated, suppresses locomotor activity, consistent with the behavioral hypersensitivity to light we observed. Our results implicate Rai1 as a novel and central player in processing non-visual light information, from input to behavioral output.
Keywords: circadian rhythm; human biology; light; medicine; mouse; neuroscience; sleep; smith-magenis syndrome; supra-chiasmatic nuclei; ventral-subparaventricular zone.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

References
-
- Bemelmans AP, Kostic C, Crippa SV, Hauswirth WW, Lem J, Munier FL, Seeliger MW, Wenzel A, Arsenijevic Y. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Medicine. 2006;3:e347. doi: 10.1371/journal.pmed.0030347. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

