Microtubules regulate brush border formation
- PMID: 28548701
- PMCID: PMC5673559
- DOI: 10.1002/jcp.26033
Microtubules regulate brush border formation
Abstract
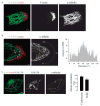
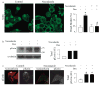
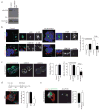
Most epithelial cells contain apical membrane structures associated to bundles of actin filaments, which constitute the brush border. Whereas microtubule participation in the maintenance of the brush border identity has been characterized, their contribution to de novo microvilli organization remained elusive. Hereby, using a cell model of individual enterocyte polarization, we found that nocodazole induced microtubule depolymerization prevented the de novo brush border formation. Microtubule participation in brush border actin organization was confirmed in polarized kidney tubule MDCK cells. We also found that centrosome, but not Golgi derived microtubules, were essential for the initial stages of brush border development. During this process, microtubule plus ends acquired an early asymmetric orientation toward the apical membrane, which clearly differs from their predominant basal orientation in mature epithelia. In addition, overexpression of the microtubule plus ends associated protein CLIP170, which regulate actin nucleation in different cell contexts, facilitated brush border formation. In combination, the present results support the participation of centrosomal microtubule plus ends in the activation of the polarized actin organization associated to brush border formation, unveiling a novel mechanism of microtubule regulation of epithelial polarity.
Keywords: MTOC; actin; brush border; epithelial polarity; microtubules.
© 2017 Wiley Periodicals, Inc.
Figures

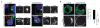


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

