Severe hypertriglyceridemia due to two novel loss-of-function lipoprotein lipase gene mutations (C310R/E396V) in a Chinese family associated with recurrent acute pancreatitis
- PMID: 28548960
- PMCID: PMC5564601
- DOI: 10.18632/oncotarget.17762
Severe hypertriglyceridemia due to two novel loss-of-function lipoprotein lipase gene mutations (C310R/E396V) in a Chinese family associated with recurrent acute pancreatitis
Abstract
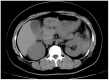
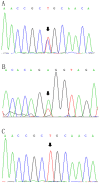
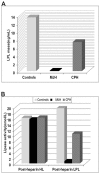
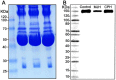
Lipoprotein lipase (LPL) is widely expressed in skeletal muscles, cardiac muscles as well as adipose tissue and involved in the catabolism of triglyceride. Herein we have systematically characterized two novel loss-of-function mutations in LPL from a Chinese family in which afflicted members were manifested by severe hypertriglyceridemia and recurrent pancreatitis. DNA sequencing revealed that the proband was a heterozygote carrying a novel c.T928C (p.C310R) mutation in exon 6 of the LPL gene. Another member of the family was detected to be a compound heterozygote who along with the c.T928C mutation also carried a novel missense mutation c.A1187T (p.E396V) in exon 8 of the LPL gene. Furthermore, COS-1 cells were transfected with lentiviruses containing the mutant LPL genes. While C310R markedly reduced the overall LPL protein level, COS-1 cells carrying E396V or double mutations contained similar overall LPL protein levels to the wild-type. The specific activity of the LPL mutants remained at comparable magnitude to the wild-type. However, few LPL were detected in the culture medium for the mutants, suggesting that both mutations caused aberrant triglyceride catabolism. More specifically, E396V and double mutations dampened the transport of LPL to the cell surface, while for the C310R mutation, reducing LPL protein level might be involved. By characterizing these two novel LPL mutations, this study has expanded our understanding on the pathogenesis of familial hypertriglyceridemia (FHTG).
Keywords: acute pancreatitis; compound heterozygosity; hypertriglyceridemia; lipoprotein lipase; mutation.
Conflict of interest statement
The authors declare no conflicts of interest
Figures





Similar articles
-
Compound but non-linked heterozygous p.W14X and p.L279 V LPL gene mutations in a Chinese patient with long-term severe hypertriglyceridemia and recurrent acute pancreatitis.Lipids Health Dis. 2018 Jun 19;17(1):144. doi: 10.1186/s12944-018-0789-2. Lipids Health Dis. 2018. PMID: 29921298 Free PMC article.
-
A novel lipoprotein lipase gene missense mutation in Chinese patients with severe hypertriglyceridemia and pancreatitis.Lipids Health Dis. 2014 Mar 19;13:52. doi: 10.1186/1476-511X-13-52. Lipids Health Dis. 2014. PMID: 24646025 Free PMC article.
-
A Chinese patient with recurrent pancreatitis during pregnancy induced by hypertriglyceridemia associated with compound heterozygosity (Glu242Lys and Leu252VaL) in the lipoprotein lipase gene.J Clin Lipidol. 2016 Jan-Feb;10(1):199-203.e1. doi: 10.1016/j.jacl.2015.09.010. Epub 2015 Sep 30. J Clin Lipidol. 2016. PMID: 26892137
-
[Hypertriglyceridemia-induced pancreatitis].Nihon Rinsho. 2013 Sep;71(9):1602-5. Nihon Rinsho. 2013. PMID: 24205721 Review. Japanese.
-
[Genetic diagnosis on hypertriglyceridemia-analysis for LPL gene mutations].Nihon Rinsho. 2013 Sep;71(9):1569-76. Nihon Rinsho. 2013. PMID: 24205716 Review. Japanese.
Cited by
-
Hypertriglyceridemia Acute Pancreatitis: Animal Experiment Research.Dig Dis Sci. 2022 Mar;67(3):761-772. doi: 10.1007/s10620-021-06928-0. Epub 2021 May 3. Dig Dis Sci. 2022. PMID: 33939144 Review.
-
Rare LPL gene missense mutation in an infant with hypertriglyceridemia.J Clin Lab Anal. 2018 Jul;32(6):e22414. doi: 10.1002/jcla.22414. Epub 2018 Feb 25. J Clin Lab Anal. 2018. PMID: 29479812 Free PMC article.
-
Loss-of-Function Homozygous Variant in LPL Causes Type I Hyperlipoproteinemia and Renal Lipidosis.Kidney Int Rep. 2023 Aug 25;8(11):2428-2438. doi: 10.1016/j.ekir.2023.08.027. eCollection 2023 Nov. Kidney Int Rep. 2023. PMID: 38025240 Free PMC article.
-
Lipoprotein Lipase: Structure, Function, and Genetic Variation.Genes (Basel). 2025 Jan 5;16(1):55. doi: 10.3390/genes16010055. Genes (Basel). 2025. PMID: 39858602 Free PMC article. Review.
-
Pseudohyponatremia: Mechanism, Diagnosis, Clinical Associations and Management.J Clin Med. 2023 Jun 15;12(12):4076. doi: 10.3390/jcm12124076. J Clin Med. 2023. PMID: 37373769 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

