The Enigmatic Role of Viruses in Multiple Sclerosis: Molecular Mimicry or Disturbed Immune Surveillance?
- PMID: 28549714
- PMCID: PMC7185415
- DOI: 10.1016/j.it.2017.04.006
The Enigmatic Role of Viruses in Multiple Sclerosis: Molecular Mimicry or Disturbed Immune Surveillance?
Abstract
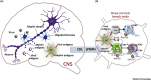
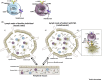
Multiple sclerosis (MS) is a T cell driven autoimmune disease of the central nervous system (CNS). Despite its association with Epstein-Barr Virus (EBV), how viral infections promote MS remains unclear. However, there is increasing evidence that the CNS is continuously surveyed by virus-specific T cells, which protect against reactivating neurotropic viruses. Here, we discuss how viral infections could lead to the breakdown of self-tolerance in genetically predisposed individuals, and how the reactivations of viruses in the CNS could induce the recruitment of both autoaggressive and virus-specific T cell subsets, causing relapses and progressive disability. A disturbed immune surveillance in MS would explain several experimental findings, and has important implications for prognosis and therapy.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

References
-
- Dendrou C.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015;15:545–558. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

