Two phosphAte taRGets in End-stage renal disease Trial (TARGET): A Randomized Controlled Trial
- PMID: 28550080
- PMCID: PMC5460712
- DOI: 10.2215/CJN.10941016
Two phosphAte taRGets in End-stage renal disease Trial (TARGET): A Randomized Controlled Trial
Abstract
Background and objectives: Hyperphosphatemia is common among recipients of maintenance dialysis and is associated with a higher risk of mortality and cardiovascular events. A large randomized trial is needed to determine whether lowering phosphate concentrations with binders improves patient-important outcomes. To inform such an effort we conducted a pilot randomized controlled trial.
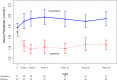
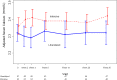
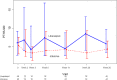
Design, setting, participants, & measurements: We conducted a randomized controlled trial of prevalent hemodialysis recipients already receiving calcium carbonate as a phosphate binder at five Canadian centers between March 31, 2014 and October 2, 2014. Participants were randomly allocated to 26 weeks of an intensive phosphate goal of 2.33-4.66 mg/dl (0.75-1.50 mmol/L) or a liberalized target of 6.20-7.75 mg/dl (2.00-2.50 mmol/L) by titrating calcium carbonate using a dosing nomogram. The primary outcome was the difference in the change in serum phosphate from randomization to 26 weeks.
Results: Fifty-three participants were randomized to the intensive group and 51 to the liberalized group. The median (interquartile range) daily dose of elemental calcium at 26 weeks was 1800 (1275-3000) mg in the intensive group, and 0 (0-500) mg in the liberalized group. The mean (SD) serum phosphate at 26 weeks was 4.53 (1.12) mg/dl (1.46 [0.36] mmol/L) in the intensive group and 6.05 (1.40) mg/dl (1.95 [0.45] mmol/L) in the liberalized group. Phosphate concentration in the intensive group declined by 1.24 (95% confidence interval, 0.75 to 1.74) mg/dl (0.40 [95% confidence interval, 0.24 to 0.56] mmol/L) compared with the liberalized group. There were no statistically significant differences between the two groups in the risk of hypercalcemia, hypocalcemia, parathyroidectomy, or major vascular events.
Conclusions: It is feasible to achieve and maintain a difference in serum phosphate concentrations in hemodialysis recipients by titrating calcium carbonate. A large trial is needed to determine if targeting a lower serum phosphate concentration improves patient-important outcomes.
Keywords: Calcium Carbonate; Calcium, Dietary; Canada; Goals; Hemodialysis; Humans; Hypercalcemia; Hypocalcemia; Kidney Failure, Chronic; Nomograms; Parathyroidectomy; Phosphates; Pilots; Prevalence; Random Allocation; hyperphosphatemia; phosphate binders; randomized controlled trials; renal dialysis; renal insufficiency, chronic.
Copyright © 2017 by the American Society of Nephrology.
Figures

Comment in
-
Getting Out of the Phosphate Bind: Trials to Guide Treatment Targets.Clin J Am Soc Nephrol. 2017 Jun 7;12(6):868-870. doi: 10.2215/CJN.04380417. Epub 2017 May 26. Clin J Am Soc Nephrol. 2017. PMID: 28550079 Free PMC article. No abstract available.
References
-
- Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 305: 1119–1127, 2011 - PubMed
-
- Block GA, Ix JH, Ketteler M, Martin KJ, Thadhani RI, Tonelli M, Wolf M, Jüppner H, Hruska K, Wheeler DC: Phosphate homeostasis in CKD: Report of a scientific symposium sponsored by the National Kidney Foundation. Am J Kidney Dis 62: 457–473, 2013 - PubMed
-
- National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 76(113): S1–S130, 2009 - PubMed
-
- Tonelli M, Pannu N, Manns B: Oral phosphate binders in patients with kidney failure. N Engl J Med 362: 1312–1324, 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

