Primary Membranous Nephropathy
- PMID: 28550082
- PMCID: PMC5460716
- DOI: 10.2215/CJN.11761116
Primary Membranous Nephropathy
Erratum in
-
Correction.Clin J Am Soc Nephrol. 2017 Sep 7;12(9):1528. doi: 10.2215/CJN.07190717. Epub 2017 Aug 3. Clin J Am Soc Nephrol. 2017. PMID: 28775127 Free PMC article. No abstract available.
Abstract
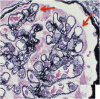
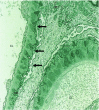
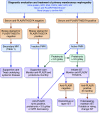
Membranous nephropathy (MN) is a unique glomerular lesion that is the most common cause of idiopathic nephrotic syndrome in nondiabetic white adults. About 80% of cases are renal limited (primary MN, PMN) and 20% are associated with other systemic diseases or exposures (secondary MN). This review focuses only on PMN. Most cases of PMN have circulating IgG4 autoantibody to the podocyte membrane antigen PLA2R (70%), biopsy evidence PLA2R staining indicating recent immunologic disease activity despite negative serum antibody levels (15%), or serum anti-THSD7A (3%-5%). The remaining 10% without demonstrable anti-PLA2R/THSd7A antibody or antigen likely have PMN probably secondary to a different, still unidentified, anti-podocyte antibody. Considerable clinical and experimental data now suggests these antibodies are pathogenic. Clinically, 80% of patients with PMN present with nephrotic syndrome and 20% with non-nephrotic proteinuria. Untreated, about one third undergo spontaneous remission, especially those with absent or low anti-PLA2R levels, one-third progress to ESRD over 10 years, and the remainder develop nonprogressive CKD. Proteinuria can persist for months after circulating anti-PLA2R/THSD7A antibody is no longer detectable (immunologic remission). All patients with PMN should be treated with supportive care from the time of diagnosis to minimize protein excretion. Patients with elevated anti-PLA2R/THSD7A levels and proteinuria >3.5 g/d at diagnosis, and those who fail to reduce proteinuria to <3.5 g after 6 months of supportive care or have complications of nephrotic syndrome, should be considered for immunosuppressive therapy. Accepted regimens include steroids/cyclophosphamide, calcineurin inhibitors, and B cell depletion. With proper management, only 10% or less will develop ESRD over the subsequent 10 years.
Keywords: Adult; B-Lymphocytes; Biopsy; Calcineurin Inhibitors; Chronic; Cyclophosphamide; Glomerulonephritis; Humans; Immunoglobulin G; Kidney Failure; Kidney Glomerulus; Lipoid; Membranous; Nephrosis; PLA2R; PLA2R1 protein; Phospholipase A2; Podocytes; Receptors; Remission; Renal Insufficiency; Spontaneous; Staining and Labeling; THSD7A; congenital; human; membranous nephropathy; nephrotic syndrome; proteinuria.
Copyright © 2017 by the American Society of Nephrology.
Figures

References
-
- Cattran DC, Brenchley PE: Membranous nephropathy: Integrating basic science into improved clinical management. Kidney Int 91: 566–574, 2017 - PubMed
-
- Salant DJ, Cattran DC: Membranous nephropathy. Chapter 20. In: Comprehensive Clinical Nephrology, 5th Ed., edited by Floege J, Johnson RJ, Feehally J, St. Louis, MI, Saunders, an imprint of Elsevier Inc., 2015, pp 239–251
-
- Kumar V, Ramachandran R, Kumar A, Nada R, Suri D, Gupta A, Kohli HS, Gupta KL, Jha V: Antibodies to m-type phospholipase A2 receptor in children with idiopathic membranous nephropathy. Nephrology (Carlton) 20: 572–575, 2015 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

