Knockdown of sodium-calcium exchanger 1 induces epithelial-to-mesenchymal transition in kidney epithelial cells
- PMID: 28550085
- PMCID: PMC5500804
- DOI: 10.1074/jbc.M116.752352
Knockdown of sodium-calcium exchanger 1 induces epithelial-to-mesenchymal transition in kidney epithelial cells
Abstract
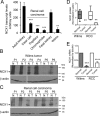
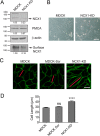
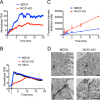
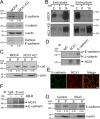
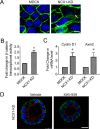
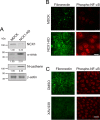
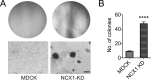
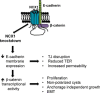
Mesenchymal-to-epithelial transition (MET) and epithelial-to-mesenchymal transition (EMT) are important processes in kidney development. Failure to undergo MET during development leads to the initiation of Wilms tumor, whereas EMT contributes to the development of renal cell carcinomas (RCC). The role of calcium regulators in governing these processes is becoming evident. We demonstrated earlier that Na+/Ca2+ exchanger 1 (NCX1), a major calcium exporter in renal epithelial cells, regulates epithelial cell motility. Here, we show for the first time that NCX1 mRNA and protein expression was down-regulated in Wilms tumor and RCC. Knockdown of NCX1 in Madin-Darby canine kidney cells induced fibroblastic morphology, increased intercellular junctional distance, and induced paracellular permeability, loss of apico-basal polarity in 3D cultures, and anchorage-independent growth, accompanied by expression of mesenchymal markers. We also provide evidence that NCX1 interacts with and anchors E-cadherin to the cell surface independent of NCX1 ion transport activity. Consistent with destabilization of E-cadherin, NCX1 knockdown cells showed an increase in β-catenin nuclear localization, enhanced transcriptional activity, and up-regulation of downstream targets of the β-catenin signaling pathway. Taken together, knockdown of NCX1 in Madin-Darby canine kidney cells alters epithelial morphology and characteristics by destabilization of E-cadherin and induction of β-catenin signaling.
Keywords: MDCK; Wilms tumor; beta-catenin (B-catenin); cadherin-1 (CDH1) (epithelial cadherin) (E-cadherin); epithelial cell; epithelial-mesenchymal transition (EMT); kidney; renal cell carcinoma; sodium-calcium exchanger.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Schedl A. (2007) Renal abnormalities and their developmental origin. Nat. Rev. Genet. 8, 791–802 - PubMed
-
- Dumanskiy Y. V., Kudriashov A. G., Vasilenko I. V., Kondratyuk R. B., Gulkov Y. K., and Cyrillichystiakov R. S. (2013) Markers of epithelial-mesenchymal transition in renal cell carcinoma. Exp. Oncol. 35, 325–327 - PubMed
-
- Harada K., Miyake H., Kusuda Y., and Fujisawa M. (2012) Expression of epithelial-mesenchymal transition markers in renal cell carcinoma: impact on prognostic outcomes in patients undergoing radical nephrectomy. BJU Int. 110, E1131–E1137 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

