Safety of Outpatient Initiation of Disopyramide for Obstructive Hypertrophic Cardiomyopathy Patients
- PMID: 28550094
- PMCID: PMC5669159
- DOI: 10.1161/JAHA.116.005152
Safety of Outpatient Initiation of Disopyramide for Obstructive Hypertrophic Cardiomyopathy Patients
Abstract
Background: Disopyramide is effective in ameliorating symptoms in patients with hypertrophic cardiomyopathy; however, its potential for proarrhythmic effect has raised concerns about its use in the ambulatory setting. The risk of initiating disopyramide in this manner has never been evaluated.
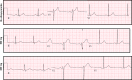
Methods and results: All charts of patients seen in the outpatient hypertrophic cardiomyopathy clinic between 2010 and 2014 were screened for initiation of disopyramide and data were extracted. Disopyramide in our clinic is usually initiated at a dose of 300 mg daily and titrated during follow-up. A total of 2015 patients were seen in the clinic, including 168 who were started on disopyramide. There were no cardiac events within 3 months of disopyramide initiation. During long-term follow-up (255 patient-years; mean, 447 days; interquartile range, 201-779), only 2 patients developed cardiac events (syncope of unknown cause in both). Thirty-eight patients (23%) developed side effects of disopyramide and 18 (11%) stopped the drug because of these side effects. Of the patients continuing disopyramide long term, 63% remained free of septal reduction interventions at end of follow-up. Disopyramide at a dose of 300 mg prolonged the mean QTc interval by 19±23 ms; however, increasing the dose to 600 mg had no further significant effect.
Conclusions: Initiation of disopyramide in the outpatient setting is safe and the risk of subsequent sudden cardiac death is low. Because of its QT-prolonging effect, precautions may be necessary in patients at higher risk of torsades de pointes.
Keywords: acquired long QT syndrome; disopyramide; hypertrophic cardiomyopathy; sudden cardiac death.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures
Similar articles
-
Disopyramide use in infants and children with hypertrophic cardiomyopathy.Cardiol Young. 2018 Apr;28(4):530-535. doi: 10.1017/S1047951117002384. Cardiol Young. 2018. PMID: 29513203
-
Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy.J Am Coll Cardiol. 2005 Apr 19;45(8):1251-8. doi: 10.1016/j.jacc.2005.01.012. J Am Coll Cardiol. 2005. PMID: 15837258
-
Heart conduction disturbances and cardiovascular collapse after disopyramide and low-dose metoprolol in a patient with hypertrophic obstructive cardiomyopathy.J Electrocardiol. 1997 Oct;30(4):341-4. doi: 10.1016/s0022-0736(97)80048-9. J Electrocardiol. 1997. PMID: 9375912
-
Effect of disopyramide on left ventricular pressure gradient in hypertrophic obstructive cardiomyopathy in comparison with propranolol--a case report.Angiology. 1999 Apr;50(4):331-5. doi: 10.1177/000331979905000410. Angiology. 1999. PMID: 10225470 Review.
-
Disopyramide for Hypertrophic Cardiomyopathy: A Pragmatic Reappraisal of an Old Drug.Pharmacotherapy. 2015 Dec;35(12):1164-72. doi: 10.1002/phar.1664. Pharmacotherapy. 2015. PMID: 26684556 Review.
Cited by
-
Hemodynamics in Left-Sided Cardiomyopathies.Rev Cardiovasc Med. 2024 Dec 24;25(12):455. doi: 10.31083/j.rcm2512455. eCollection 2024 Dec. Rev Cardiovasc Med. 2024. PMID: 39742240 Free PMC article. Review.
-
Electrophysiological and Contractile Effects of Disopyramide in Patients With Obstructive Hypertrophic Cardiomyopathy: A Translational Study.JACC Basic Transl Sci. 2019 Oct 9;4(7):795-813. doi: 10.1016/j.jacbts.2019.06.004. eCollection 2019 Nov. JACC Basic Transl Sci. 2019. PMID: 31998849 Free PMC article.
-
Disopyramide Revisited for Treatment of Symptomatic Obstructive Hypertrophic Cardiomyopathy: Efficacy and Safety in Patients Treated for at Least 5 Years.J Am Heart Assoc. 2025 Jan 21;14(2):e037639. doi: 10.1161/JAHA.124.037639. Epub 2025 Jan 16. J Am Heart Assoc. 2025. PMID: 39817530 Free PMC article.
-
Tailored Therapies for Cardiogenic Shock in Hypertrophic Cardiomyopathy: Navigating Emerging Strategies.J Cardiovasc Dev Dis. 2024 Dec 11;11(12):401. doi: 10.3390/jcdd11120401. J Cardiovasc Dev Dis. 2024. PMID: 39728291 Free PMC article. Review.
-
Cardiac myosin inhibitors: Efficacy, safety and future directions of aficamten in hypertrophic obstructive cardiomyopathy.Egypt Heart J. 2025 Jun 13;77(1):61. doi: 10.1186/s43044-025-00652-0. Egypt Heart J. 2025. PMID: 40512253 Free PMC article. Review.
References
-
- Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–789. - PubMed
-
- Shah JS, Esteban MT, Thaman R, Sharma R, Mist B, Pantazis A, Ward D, Kohli SK, Page SP, Demetrescu C, Sevdalis E, Keren A, Pellerin D, McKenna WJ, Elliott PM. Prevalence of exercise‐induced left ventricular outflow tract obstruction in symptomatic patients with non‐obstructive hypertrophic cardiomyopathy. Heart. 2008;94:1288–1294. - PubMed
-
- Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–255. - PubMed
-
- Sherrid MV, Shetty A, Winson G, Kim B, Musat D, Alviar CL, Homel P, Balaram SK, Swistel DG. Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first‐line therapy with beta‐blockade or verapamil. Circ Heart Fail. 2013;6:694–702. - PubMed
-
- Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

