A novel experimental rat model of peripheral nerve scarring that reliably mimics post-surgical complications and recurring adhesions
- PMID: 28550101
- PMCID: PMC5560061
- DOI: 10.1242/dmm.028852
A novel experimental rat model of peripheral nerve scarring that reliably mimics post-surgical complications and recurring adhesions
Abstract
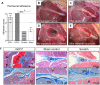
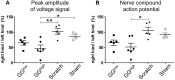
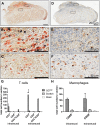
Inflammation, fibrosis and perineural adhesions with the surrounding tissue are common pathological processes following nerve injury and surgical interventions on peripheral nerves in human patients. These features can reoccur following external neurolysis, currently the most common surgical treatment for peripheral nerve scarring, thus leading to renewed nerve function impairment and chronic pain. To enable a successful evaluation of new therapeutic approaches, it is crucial to use a reproducible animal model that mimics the main clinical symptoms occurring in human patients. However, a clinically relevant model combining both histological and functional alterations has not been published to date. We therefore developed a reliable rat model that exhibits the essential pathological processes of peripheral nerve scarring. In our study, we present a novel method for the induction of nerve scarring by applying glutaraldehyde-containing glue that is known to cause nerve injury in humans. After a 3-week contact period with the sciatic nerve in female Sprague Dawley rats, we could demonstrate severe intra- and perineural scarring that resulted in grade 3 adhesions and major impairments in the electrophysiological peak amplitude compared with sham control (P=0.0478). Immunohistochemical analysis of the nerve structure revealed vigorous nerve inflammation and recruitment of T cells and macrophages. Also, distinct nerve degeneration was determined by immunostaining. These pathological alterations were further reflected in significant functional deficiencies, as determined by the analysis of relevant gait parameters as well as the quantification of the sciatic functional index starting at week 1 post-operation (P<0.01). Moreover, with this model we could, for the first time, demonstrate not only the primary formation, but also the recurrence, of severe adhesions 1 week after glue removal, imitating a major clinical challenge. As a comparison, we tested a published model for generating perineural fibrotic adhesions, which did not result in significant pathological changes. Taken together, we established an easily reproducible and reliable rat model for peripheral nerve scarring that allows for the effective testing of new therapeutic strategies.
Keywords: Nerve fibrosis; Nerve inflammation; Nerve scarring; Perineural adhesions; Peripheral nerve adhesions.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Albayrak B. S., Ismailoglu O., Ilbay K., Yaka U., Tanriover G., Gorgulu A. and Demir N. (2010). Doxorubicin for prevention of epineurial fibrosis in a rat sciatic nerve model: outcome based on gross postsurgical, histopathological, and ultrastructural findings Laboratory investigation. J. Neurosurg. Spine 12, 327-333. 10.3171/2009.9.SPINE09407 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

