Hypothalamic Ventromedial Lin28a Enhances Glucose Metabolism in Diet-Induced Obesity
- PMID: 28550108
- PMCID: PMC5521863
- DOI: 10.2337/db16-1558
Hypothalamic Ventromedial Lin28a Enhances Glucose Metabolism in Diet-Induced Obesity
Abstract
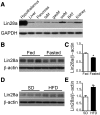
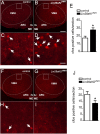
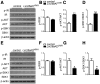
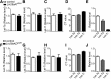
The Lin28a/Let-7 axis has been studied in peripheral tissues for its role in metabolism regulation. However, its central function remains unclear. Here we found that Lin28a is highly expressed in the hypothalamus compared with peripheral tissues. Its expression is positively correlated with positive energy balance, suggesting a potential central role for Lin28a in metabolism regulation. Thus, we targeted the hypothalamic ventromedial nucleus (VMH) to selectively overexpress (Lin28aKIVMH ) or downregulate (Lin28aKDVMH ) Lin28a expression in mice. With mice on a standard chow diet, body weight and glucose homeostasis were not affected in Lin28aKIVMH or Lin28aKDVMH mice. On a high-fat diet, although no differences in body weight and composition were observed, Lin28aKIVMH mice showed improved glucose tolerance and insulin sensitivity compared with controls. Conversely, Lin28aKDVMH mice displayed glucose intolerance and insulin resistance. Changes in VMH AKT activation of diet-induced obese Lin28aKIVMH or Lin28aKDVMH mice were not associated with alterations in Let-7 levels or insulin receptor activation. Rather, we observed altered expression of TANK-binding kinase-1 (TBK-1), which was found to be a direct Lin28a target mRNA. VMH-specific inhibition of TBK-1 in mice with diet-induced obesity impaired glucose metabolism and AKT activation. Altogether, our data show a TBK-1-dependent role for central Lin28a in glucose homeostasis.
© 2017 by the American Diabetes Association.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

