Host-Derived CD70 Suppresses Murine Graft-versus-Host Disease by Limiting Donor T Cell Expansion and Effector Function
- PMID: 28550198
- PMCID: PMC5503479
- DOI: 10.4049/jimmunol.1502181
Host-Derived CD70 Suppresses Murine Graft-versus-Host Disease by Limiting Donor T Cell Expansion and Effector Function
Abstract
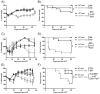
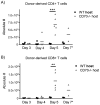
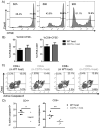
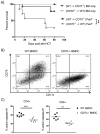
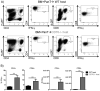
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment for hematologic and immunologic diseases. However, graft-versus-host disease (GVHD) may develop when donor-derived T cells recognize and damage genetically distinct normal host tissues. In addition to TCR signaling, costimulatory pathways are involved in T cell activation. CD27 is a TNFR family member expressed on T cells, and its ligand, CD70, is expressed on APCs. The CD27/CD70 costimulatory pathway was shown to be critical for T cell function and survival in viral infection models. However, the role of this pathway in allo-HCT is previously unknown. In this study, we have examined its contribution in GVHD pathogenesis. Surprisingly, Ab blockade of CD70 after allo-HCT significantly increases GVHD. Interestingly, whereas donor T cell- or bone marrow-derived CD70 plays no role in GVHD, host-derived CD70 inhibits GVHD as CD70-/- hosts show significantly increased GVHD. This is evidenced by reduced survival, more severe weight loss, and increased histopathologic damage compared with wild-type hosts. In addition, CD70-/- hosts have higher levels of proinflammatory cytokines TNF-α, IFN-γ, IL-2, and IL-17. Moreover, accumulation of donor CD4+ and CD8+ effector T cells is increased in CD70-/- versus wild-type hosts. Mechanistic analyses suggest that CD70 expressed by host hematopoietic cells is involved in the control of alloreactive T cell apoptosis and expansion. Together, our findings demonstrate that host CD70 serves as a unique negative regulator of allogeneic T cell response by contributing to donor T cell apoptosis and inhibiting expansion of donor effector T cells.
Copyright © 2017 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures




References
-
- Gandy KL, Domen J, Aguila H, Weissman IL. CD8+TCR+ and CD8+TCR- cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity. 1999;11:579–590. - PubMed
-
- Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, Carabasi MH, Gale RP, Giralt S, Hale GA, Ilhan O, McCarthy PL, Socie G, Verdonck LF, Weisdorf DJ, Horowitz MM. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–414. - PubMed
-
- Chakrabarti S, Mautner V, Osman H, Collingham KE, Fegan CD, Klapper PE, Moss PA, Milligan DW. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100:1619–1627. - PubMed
-
- Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

