Comprehensive Approach for Identifying the T Cell Subset Origin of CD3 and CD28 Antibody-Activated Chimeric Antigen Receptor-Modified T Cells
- PMID: 28550199
- PMCID: PMC5536854
- DOI: 10.4049/jimmunol.1601494
Comprehensive Approach for Identifying the T Cell Subset Origin of CD3 and CD28 Antibody-Activated Chimeric Antigen Receptor-Modified T Cells
Abstract
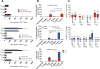
The outcome of therapy with chimeric Ag receptor (CAR)-modified T cells is strongly influenced by the subset origin of the infused T cells. However, because polyclonally activated T cells acquire a largely CD45RO+CCR7- effector memory phenotype after expansion, regardless of subset origin, it is impossible to know which subsets contribute to the final T cell product. To determine the contribution of naive T cell, memory stem T cell, central memory T cell, effector memory T cell, and terminally differentiated effector T cell populations to the CD3 and CD28-activated CAR-modified T cells that we use for therapy, we followed the fate and function of individually sorted CAR-modified T cell subsets after activation with CD3 and CD28 Abs (CD3/28), transduction and culture alone, or after reconstitution into the relevant subset-depleted population. We show that all subsets are sensitive to CAR transduction, and each developed a distinct T cell functional profile during culture. Naive-derived T cells showed the greatest rate of proliferation but had more limited effector functions and reduced killing compared with memory-derived populations. When cultured in the presence of memory T cells, naive-derived T cells show increased differentiation, reduced effector cytokine production, and a reduced reproliferative response to CAR stimulation. CD3/28-activated T cells expanded in IL-7 and IL-15 produced greater expansion of memory stem T cells and central memory T cell-derived T cells compared with IL-2. Our strategy provides a powerful tool to elucidate the characteristics of CAR-modified T cells, regardless of the protocol used for expansion, reveals the functional properties of each expanded T cell subset, and paves the way for a more detailed evaluation of the effects of manufacturing changes on the subset contribution to in vitro-expanded T cells.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures

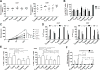



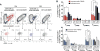


References
-
- Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, Liu H, Grilley B, Rooney CM, Heslop HE, Brenner MK, Dotti G. Brief report CD28 costimulation improves expansion and persistence of chimeric antigen receptor – modified T cells in lymphoma patients. 2011;121:1–5. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

