Development of a candidate stabilizing formulation for bulk storage of a double mutant heat labile toxin (dmLT) protein based adjuvant
- PMID: 28551040
- PMCID: PMC5628956
- DOI: 10.1016/j.vaccine.2017.03.101
Development of a candidate stabilizing formulation for bulk storage of a double mutant heat labile toxin (dmLT) protein based adjuvant
Abstract
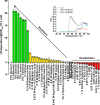
This work describes the formulation design and development of a novel protein based adjuvant, a double mutant of heat labile toxin (dmLT), based on knowledge of the protein's structural integrity and physicochemical degradation pathways. Various classes of pharmaceutical excipients were screened for their stabilizing effect on dmLT during exposure to thermal and agitation stresses as monitored by high throughput analytical assays for dmLT degradation. Sucrose, phosphate, sodium chloride, methionine and polysorbate-80 were identified as potential stabilizers that protected dmLT against either conformational destabilization, aggregation/particle formation or chemical degradation (e.g., Met oxidation and Lys glycation). Different combinations and concentrations of the selected stabilizers were then evaluated to further optimize dmLT stability while maintaining pharmaceutically acceptable ranges of solution pH and osmolality. The effect of multiple freeze-thaw (FT) cycles on the physical stability of candidate bulk formulations was also examined. Increasing the polysorbate-80 concentration to 0.1% in the lead candidate bulk formulation mitigated the loss of protein mass during FT. This formulation development study enabled the design of a new bulk formulation of the dmLT adjuvant and provides flexibility for future use in combination with a variety of different vaccine dosage forms with different antigens.
Keywords: Adjuvant; Aggregation; Excipient; Formulation; Stability; dmLT.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Reed S.G., Bertholet S., Coler R.N., Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. - PubMed
-
- Petrovsky N., Aguilar J.C. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–496. - PubMed
-
- Brito L.A., Malyala P., O’Hagan D.T. Vaccine adjuvant formulations: a pharmaceutical perspective. Semin Immunol. 2013;25:130–145. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

