Bi-directionally protective communication between neurons and astrocytes under ischemia
- PMID: 28551085
- PMCID: PMC5447396
- DOI: 10.1016/j.redox.2017.05.010
Bi-directionally protective communication between neurons and astrocytes under ischemia
Abstract
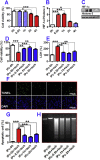
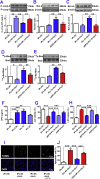
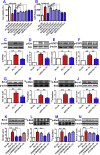
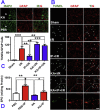
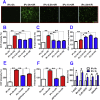
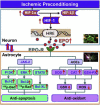
The extensive existing knowledge on bi-directional communication between astrocytes and neurons led us to hypothesize that not only ischemia-preconditioned (IP) astrocytes can protect neurons but also IP neurons protect astrocytes from lethal ischemic injury. Here, we demonstrated for the first time that neurons have a significant role in protecting astrocytes from ischemic injury. The cultured medium from IP neurons (IPcNCM) induced a remarkable reduction in LDH and an increase in cell viability in ischemic astrocytes in vitro. Selective neuronal loss by kainic acid injection induced a significant increase in apoptotic astrocyte numbers in the brain of ischemic rats in vivo. Furthermore, TUNEL analysis, DNA ladder assay, and the measurements of ROS, GSH, pro- and anti-apoptotic factors, anti-oxidant enzymes and signal molecules in vitro and/or in vivo demonstrated that IP neurons protect astrocytes by an EPO-mediated inhibition of pro-apoptotic signals, activation of anti-apoptotic proteins via the P13K/ERK/STAT5 pathways and activation of anti-oxidant proteins via up-regulation of anti-oxidant enzymes. We demonstrated the existence of astro-protection by IP neurons under ischemia and proposed that the bi-directionally protective communications between cells might be a common activity in the brain or peripheral organs under most if not all pathological conditions.
Keywords: Anti-apoptosis and anti-oxidant; Astro-protection; Bi-directional communication.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
The involvement of nuclear factor-κB in astroprotection against ischemia-reperfusion injury by ischemia-preconditioned neurons.J Cell Physiol. 2021 Jun;236(6):4515-4527. doi: 10.1002/jcp.30168. Epub 2021 Jan 14. J Cell Physiol. 2021. PMID: 33442879
-
Ginkgolide B preconditioning on astrocytes promotes neuronal survival in ischemic injury via up-regulating erythropoietin secretion.Neurochem Int. 2013 Jan;62(2):157-64. doi: 10.1016/j.neuint.2012.11.007. Epub 2012 Nov 28. Neurochem Int. 2013. PMID: 23201340
-
Hyperthermia conditioned astrocyte-cultured medium protects neurons from ischemic injury by the up-regulation of HIF-1 alpha and the increased anti-apoptotic ability.Eur J Pharmacol. 2011 Sep;666(1-3):19-25. doi: 10.1016/j.ejphar.2011.05.018. Epub 2011 May 23. Eur J Pharmacol. 2011. PMID: 21620821
-
Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species.Eur J Biochem. 2000 Aug;267(16):4912-6. doi: 10.1046/j.1432-1327.2000.01597.x. Eur J Biochem. 2000. PMID: 10931173 Review.
-
[Apoptosis of astroglial cells].Nihon Yakurigaku Zasshi. 1998 Oct;112 Suppl 1:24P-27P. doi: 10.1254/fpj.112.supplement_24. Nihon Yakurigaku Zasshi. 1998. PMID: 10190127 Review. Japanese.
Cited by
-
Erythropoietin as a Neuroprotective Molecule: An Overview of Its Therapeutic Potential in Neurodegenerative Diseases.ASN Neuro. 2019 Jan-Dec;11:1759091419871420. doi: 10.1177/1759091419871420. ASN Neuro. 2019. PMID: 31450955 Free PMC article. Review.
-
NRF1-mediated microglial activation triggers high-altitude cerebral edema.J Mol Cell Biol. 2022 Sep 19;14(5):mjac036. doi: 10.1093/jmcb/mjac036. J Mol Cell Biol. 2022. PMID: 35704676 Free PMC article.
-
AXL kinase-mediated astrocytic phagocytosis modulates outcomes of traumatic brain injury.J Neuroinflammation. 2021 Jul 7;18(1):154. doi: 10.1186/s12974-021-02201-3. J Neuroinflammation. 2021. PMID: 34233703 Free PMC article.
-
Melatonin Prevents Mice Cortical Astrocytes From Hemin-Induced Toxicity Through Activating PKCα/Nrf2/HO-1 Signaling in vitro.Front Neurosci. 2019 Jul 25;13:760. doi: 10.3389/fnins.2019.00760. eCollection 2019. Front Neurosci. 2019. PMID: 31404262 Free PMC article.
-
CX3CL1/CX3CR1 signal mediates M1-type microglia and accelerates high-altitude-induced forgetting.Front Cell Neurosci. 2023 May 10;17:1189348. doi: 10.3389/fncel.2023.1189348. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37234914 Free PMC article.
References
-
- Martin L.J., Brambrink A.M., Lehmann C., Portera-Cailliau C., Koehler R., Rothstein J., Traystman R.J. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann. Neurol. 1997;42:335–348. - PubMed
-
- Gidday J.M. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci. 2006;7:437–448. - PubMed
-
- Murray C.E., Jennings R.B., Reimer K.A. Preconditioning with ischaemia: a delay of lethal cell injury in ischaemic myocardium. Circulation. 1986;74:1124–1136. - PubMed
-
- Hausenloy D.J., Yellon D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016;13:193–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

