A unique mid-sequence linker used to multimerize the lipid-phosphatidylserine (PS) binding peptide-peptoid hybrid PPS1
- PMID: 28551176
- PMCID: PMC5555622
- DOI: 10.1016/j.ejmech.2017.05.040
A unique mid-sequence linker used to multimerize the lipid-phosphatidylserine (PS) binding peptide-peptoid hybrid PPS1
Abstract
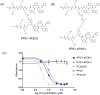
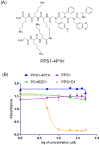
Ligand multimerizations enhance the binding affinity towards cell surface biomarkers through their avidity effects. Typical linkers connect individual monomeric ligand moieties from one end (e.g., C- or N-terminus of a peptide) and exclusively target protein receptors. The lipid phosphatidylserine (PS) is normally present on the cytoplasmic side of the eukaryotic cell membrane, but in tumors and tumor endothelial cells, this negatively charged PS flips to the outer layer. We recently reported a PS binding peptide-peptoid hybrid (PPS1) that has distinct positively charged and hydrophobic residue-containing regions. The PPS1 monomer is inactive, and upon C-terminal dimerization (PPS1D1), it triggers cytotoxicity. In the current study, a unique series of PPS1 multimeric derivatives were synthesized by switching the linker from the C-terminus to an internal position. The unimportant fourth residue (N-lys) from the C-terminus was utilized to build the linker. The synthesis strategy was developed employing variations of (I) the linker size, (II) the number of positively charged residues, and (III) the number of hydrophobic regions. Cytotoxicity of these new derivatives on HCC4017 lung cancer cells showed that a minimum of two hydrophobic regions was important to retain the activity and that the shortest linker length was optimal for activity.
Keywords: Multimers; Peptoids; Sequence modifications.
Copyright © 2017 Elsevier Masson SAS. All rights reserved.
Figures




Similar articles
-
A mini-library system to investigate non-essential residues of lipid-phosphatidylserine (PS) binding peptide-peptoid hybrid PPS1.Medchemcomm. 2017 Dec 1;8(12):2208-2215. doi: 10.1039/C7MD00372B. Epub 2017 Oct 24. Medchemcomm. 2017. PMID: 29527284 Free PMC article.
-
A comprehensive lipid binding and activity validation of a cancer-specific peptide-peptoid hybrid PPS1.Biochem Biophys Res Commun. 2017 Apr 29;486(2):545-550. doi: 10.1016/j.bbrc.2017.03.083. Epub 2017 Mar 18. Biochem Biophys Res Commun. 2017. PMID: 28322795 Free PMC article.
-
Identification of the minimum pharmacophore of lipid-phosphatidylserine (PS) binding peptide-peptoid hybrid PPS1D1.Bioorg Med Chem. 2016 Sep 15;24(18):4470-4477. doi: 10.1016/j.bmc.2016.07.045. Epub 2016 Jul 21. Bioorg Med Chem. 2016. PMID: 27485601 Free PMC article.
-
Identification of lipid-phosphatidylserine (PS) as the target of unbiasedly selected cancer specific peptide-peptoid hybrid PPS1.Oncotarget. 2016 May 24;7(21):30678-90. doi: 10.18632/oncotarget.8929. Oncotarget. 2016. PMID: 27120792 Free PMC article.
-
Phosphatidylserine: A cancer cell targeting biomarker.Semin Cancer Biol. 2018 Oct;52(Pt 1):17-25. doi: 10.1016/j.semcancer.2017.08.012. Epub 2017 Sep 1. Semin Cancer Biol. 2018. PMID: 28870843 Review.
Cited by
-
A mini-library system to investigate non-essential residues of lipid-phosphatidylserine (PS) binding peptide-peptoid hybrid PPS1.Medchemcomm. 2017 Dec 1;8(12):2208-2215. doi: 10.1039/C7MD00372B. Epub 2017 Oct 24. Medchemcomm. 2017. PMID: 29527284 Free PMC article.
-
Peptidomimetic Oligomers Targeting Membrane Phosphatidylserine Exhibit Broad Antiviral Activity.ACS Infect Dis. 2023 Aug 11;9(8):1508-1522. doi: 10.1021/acsinfecdis.3c00063. Epub 2023 Aug 2. ACS Infect Dis. 2023. PMID: 37530426 Free PMC article.
-
A novel peptidomimetic therapeutic for selective suppression of lung cancer stem cells over non-stem cancer cells.Bioorg Chem. 2021 Nov;116:105340. doi: 10.1016/j.bioorg.2021.105340. Epub 2021 Sep 8. Bioorg Chem. 2021. PMID: 34530236 Free PMC article.
-
Unbiased peptoid cell screen identifies a peptoid targeting newly appeared cell surface vimentin on tumor transformed early lung cancer cells.Bioorg Med Chem. 2022 Mar 15;58:116673. doi: 10.1016/j.bmc.2022.116673. Epub 2022 Feb 15. Bioorg Med Chem. 2022. PMID: 35189561 Free PMC article.
References
-
- Feng D, Shaikh AS, Wang F. Recent Advance in Tumor-associated Carbohydrate Antigens (TACAs)-based Antitumor Vaccines. ACS Chem Biol. 2016;11:850–863. - PubMed
-
- Ruzza P, Marchiani A, Antolini N, Calderan A. Peptide-receptor ligands and multivalent approach. Anticancer Agents Med Chem. 2012;12:416–427. - PubMed
-
- Galan MC, Dumy P, Renaudet O. Multivalent glyco(cyclo)peptides. Chem Soc Rev. 2013;42:4599–4612. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

