A Novel Platform for Immune Tolerance Induction in Hemophilia A Mice
- PMID: 28552407
- PMCID: PMC5542771
- DOI: 10.1016/j.ymthe.2017.04.029
A Novel Platform for Immune Tolerance Induction in Hemophilia A Mice
Abstract
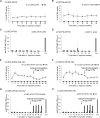
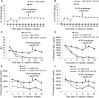
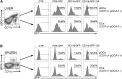
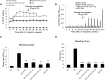
Hemophilia A (HA) is an X-linked bleeding disease caused by factor VIII (FVIII) deficiency. We previously demonstrated that FVIII is produced specifically in liver sinusoid endothelial cells (LSECs) and to some degree in myeloid cells, and thus, in the present work, we seek to restrict the expression of FVIII transgene to these cells using cell-specific promoters. With this approach, we aim to limit immune response in a mouse model by lentiviral vector (LV)-mediated gene therapy encoding FVIII. To increase the target specificity of FVIII expression, we included miRNA target sequences (miRTs) (i.e., miRT-142.3p, miRT-126, and miRT-122) to silence expression in hematopoietic cells, endothelial cells, and hepatocytes, respectively. Notably, we report, for the first time, therapeutic levels of FVIII transgene expression at its natural site of production, which occurred without the formation of neutralizing antibodies (inhibitors). Moreover, inhibitors were eradicated in FVIII pre-immune mice through a regulatory T cell-dependent mechanism. In conclusion, targeting FVIII expression to LSECs and myeloid cells by using LVs with cell-specific promoter minimized off-target expression and immune responses. Therefore, at least for some transgenes, expression at the physiologic site of synthesis can enhance efficacy and safety, resulting in long-term correction of genetic diseases such as HA.
Keywords: Tregs; gene therapy; hemophilia A; inhibitor titers reversion; targeted FVIII expression.
Copyright © 2017. Published by Elsevier Inc.
Figures




Comment in
-
Micromanaging Tolerance in Hemophilia A Gene Therapy.Mol Ther. 2017 Aug 2;25(8):1739-1740. doi: 10.1016/j.ymthe.2017.06.001. Epub 2017 Jun 16. Mol Ther. 2017. PMID: 28625571 Free PMC article. No abstract available.
References
-
- Bolton-Maggs P.H., Pasi K.J. Haemophilias A and B. Lancet. 2003;361:1801–1809. - PubMed
-
- Graw J., Brackmann H.H., Oldenburg J., Schneppenheim R., Spannagl M., Schwaab R. Haemophilia A: from mutation analysis to new therapies. Nat. Rev. Genet. 2005;6:488–501. - PubMed
-
- Roth D.A., Tawa N.E., Jr., O’Brien J.M., Treco D.A., Selden R.F., Factor V.T.T.S.G., Factor VIII Transkaryotic Therapy Study Group Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N. Engl. J. Med. 2001;344:1735–1742. - PubMed
-
- Ishiwata A., Mimuro J., Mizukami H., Kashiwakura Y., Takano K., Ohmori T., Madoiwa S., Ozawa K., Sakata Y. Liver-restricted expression of the canine factor VIII gene facilitates prevention of inhibitor formation in factor VIII-deficient mice. J. Gene Med. 2009;11:1020–1029. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

