Effects of force detecting sense organs on muscle synergies are correlated with their response properties
- PMID: 28552666
- PMCID: PMC5817982
- DOI: 10.1016/j.asd.2017.05.004
Effects of force detecting sense organs on muscle synergies are correlated with their response properties
Abstract
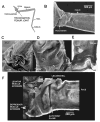
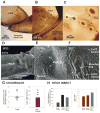
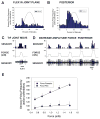
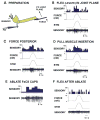
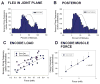
Sense organs that monitor forces in legs can contribute to activation of muscles as synergist groups. Previous studies in cockroaches and stick insects showed that campaniform sensilla, receptors that encode forces via exoskeletal strains, enhance muscle synergies in substrate grip. However synergist activation was mediated by different groups of receptors in cockroaches (trochanteral sensilla) and stick insects (femoral sensilla). The factors underlying the differential effects are unclear as the responses of femoral campaniform sensilla have not previously been characterized. The present study characterized the structure and response properties (via extracellular recording) of the femoral sensilla in both insects. The cockroach trochantero-femoral (TrF) joint is mobile and the joint membrane acts as an elastic antagonist to the reductor muscle. Cockroach femoral campaniform sensilla show weak discharges to forces in the coxo-trochanteral (CTr) joint plane (in which forces are generated by coxal muscles) but instead encode forces directed posteriorly (TrF joint plane). In stick insects, the TrF joint is fused and femoral campaniform sensilla discharge both to forces directed posteriorly and forces in the CTr joint plane. These findings support the idea that receptors that enhance synergies encode forces in the plane of action of leg muscles used in support and propulsion.
Keywords: Campaniform; Force; Insect; Response; Sensitivity; Synergies.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures



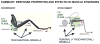
Similar articles
-
Force feedback reinforces muscle synergies in insect legs.Arthropod Struct Dev. 2015 Nov;44(6 Pt A):541-53. doi: 10.1016/j.asd.2015.07.001. Epub 2015 Jul 17. Arthropod Struct Dev. 2015. PMID: 26193626
-
Force encoding in stick insect legs delineates a reference frame for motor control.J Neurophysiol. 2012 Sep;108(5):1453-72. doi: 10.1152/jn.00274.2012. Epub 2012 Jun 6. J Neurophysiol. 2012. PMID: 22673329 Free PMC article.
-
Force dynamics and synergist muscle activation in stick insects: the effects of using joint torques as mechanical stimuli.J Neurophysiol. 2018 Oct 1;120(4):1807-1823. doi: 10.1152/jn.00371.2018. Epub 2018 Jul 18. J Neurophysiol. 2018. PMID: 30020837 Free PMC article.
-
The scolopidial accessory organs and Nebenorgans in orthopteroid insects: Comparative neuroanatomy, mechanosensory function, and evolutionary origin.Arthropod Struct Dev. 2017 Nov;46(6):765-776. doi: 10.1016/j.asd.2017.08.004. Epub 2017 Sep 19. Arthropod Struct Dev. 2017. PMID: 28864301 Review.
-
The evolution of insect wings and their sensory apparatus.Brain Behav Evol. 1997 Jul;50(1):13-24. doi: 10.1159/000113318. Brain Behav Evol. 1997. PMID: 9209763 Review.
Cited by
-
Ultra high-resolution biomechanics suggest that substructures within insect mechanosensors decisively affect their sensitivity.J R Soc Interface. 2022 May;19(190):20220102. doi: 10.1098/rsif.2022.0102. Epub 2022 May 4. J R Soc Interface. 2022. PMID: 35506211 Free PMC article.
-
Identification of the origin of force-feedback signals influencing motor neurons of the thoraco-coxal joint in an insect.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2019 Apr;205(2):253-270. doi: 10.1007/s00359-019-01334-4. Epub 2019 Apr 11. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2019. PMID: 30976919
-
Thorax-Segment- and Leg-Segment-Specific Motor Control for Adaptive Behavior.Front Physiol. 2022 May 4;13:883858. doi: 10.3389/fphys.2022.883858. eCollection 2022. Front Physiol. 2022. PMID: 35600292 Free PMC article.
-
Snow flies self-amputate freezing limbs to sustain behavior at sub-zero temperatures.Curr Biol. 2023 Nov 6;33(21):4549-4556.e3. doi: 10.1016/j.cub.2023.09.002. Epub 2023 Sep 26. Curr Biol. 2023. PMID: 37757830 Free PMC article.
-
A load-based mechanism for inter-leg coordination in insects.Proc Biol Sci. 2017 Dec 13;284(1868):20171755. doi: 10.1098/rspb.2017.1755. Proc Biol Sci. 2017. PMID: 29187626 Free PMC article.
References
-
- Akay T, Bässler U, Gerharz P, Büschges A. The role of sensory signals from the insect coxa-trochanteral joint in controlling motor activity of the femur-tibia joint. J Neurophysiol. 2001;85:594–604. - PubMed
-
- Akay T. Ph D Thesis. University of Cologne; 2002. The Role of Sensory Signals for Interjoint Coordination in Stick Insect Legs (Carausius morosus and Cuniculina impigra)
-
- Ayali A, Borgmann A, Büschges A, Couzin-Fuchs E, Daun-Gruhn S, Holmes P. The comparative investigation of the stick insect and cockroach models in the study of insect locomotion. Curr Opin Insect Sci. 2015;12:1–10.
-
- Bässler U. Neural Basis of Elementary Behavior in Stick Insects. Springer; Berlin: 1983.
-
- Bässler U, Rohrbacher J, Karg G, Breutel G. Interruption of searching movements of partly restrained front legs of stick insects, a model situation for the start of a stance phase? Biol Cybern. 1991;65:507–514.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

