Selection of viable in vitro-fertilized bovine embryos using time-lapse monitoring in microwell culture dishes
- PMID: 28552887
- PMCID: PMC5593086
- DOI: 10.1262/jrd.2017-041
Selection of viable in vitro-fertilized bovine embryos using time-lapse monitoring in microwell culture dishes
Abstract
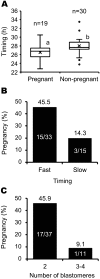
Conventionally, in vitro-fertilized (IVF) bovine embryos for transfer are morphologically evaluated at day 7-8 of embryo culture. This method is, however, subjective and results in unreliable selection. We previously described a novel selection system for IVF bovine blastocysts for transfer that traces the development of individual embryos with time-lapse monitoring in our specially developed microwell culture dishes (LinKID micro25). The system can noninvasively identify prognostic factors that reflect viability after transfer. By assessing a combination of identified prognostic factors -timing of the first cleavage; number of blastomeres at the end of the first cleavage; and number of blastomeres at the onset of lag-phase, which results in temporary developmental arrest during the fourth or fifth cell cycle- the pregnancy rate was improved over using conventional morphological evaluation. Time-lapse monitoring with LinKID micro25 could facilitate objective and reliable selection of healthy IVF bovine embryos. Here, we review the novel bovine embryo selection system that allows for prediction of viability after transfer.
Keywords: Bovine embryo; Culture dish; Pregnancy; Selection; Time-lapse monitoring.
Figures
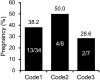

References
-
- 1.Stringfellow DA, Seidel SM, IETS. IETS manual. Manual of the International Embryo Transfer Society, 2001.
-
- Farin PW, Britt JH, Shaw DW, Slenning BD. Agreement among evaluators of bovine embryos produced in vivo or in vitro. Theriogenology 1995; 44: 339–349. - PubMed
-
- Koo DB, Kang YK, Choi YH, Park JS, Kim HN, Oh KB, Son DS, Park H, Lee KK, Han YM. Aberrant allocations of inner cell mass and trophectoderm cells in bovine nuclear transfer blastocysts. Biol Reprod 2002; 67: 487–492. - PubMed
-
- Gjørret JO, Knijn HM, Dieleman SJ, Avery B, Larsson LI, Maddox-Hyttel P. Chronology of apoptosis in bovine embryos produced in vivo and in vitro. Biol Reprod 2003; 69: 1193–1200. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

