Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis?
- PMID: 28552897
- PMCID: PMC5517538
- DOI: 10.5551/jat.RV17006
Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis?
Abstract
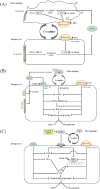
Intestinal flora (microbiota) have recently attracted attention among lipid and carbohydrate metabolism researchers. Microbiota metabolize resistant starches and dietary fibers through fermentation and decomposition, and provide short chain fatty acids (SCFAs) to the host. The major SCFAs acetates, propionate and butyrate, have different production ratios and physiological activities. Several receptors for SCFAs have been identified as the G-protein coupled receptor 41/free fatty acid receptor 3 (GPR41/FFAR3), GPR43/FFAR2, GPR109A, and olfactory receptor 78, which are present in intestinal epithelial cells, immune cells, and adipocytes, despite their expression levels differing between tissues and cell types. Many studies have indicated that SCFAs exhibit a wide range of functions from immune regulation to metabolism in a variety of tissues and organs, and therefore have both a direct and indirect influence on our bodies. This review will focus on SCFAs, especially butyrate, and their effects on various inflammatory mechanisms including atherosclerosis. In the future, SCFAs may provide new insights into understanding the pathophysiology of chronic inflammation, metabolic disorders, and atherosclerosis, and we can expect the development of novel therapeutic strategies for these diseases.
Keywords: Atherosclerosis; Butyrate; Inflammation; Microbiota; Short chain fatty acids.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures
References
-
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Meta-HIT Consortium, Bork P, Ehrlich SD, Wang J: A human gut microbial gene catalogue established by metagenomic sequencing. Nature, 2010; 464: 59-65 - PMC - PubMed
-
- Lepage P, Leclerc MC, Joossens M, Mondot S, Blottière HM, Raes J, Ehrlich D, Doré J: A metagenomic insight into our gut's microbiome. Gut, 2013; 62: 146-158 - PubMed
-
- Macfarlane GT, Macfarlane S: Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int, 2012; 95: 50-60 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

