Skeletal and Appendage Diversity as Design Elements in the Synthesis of a Discovery Library of Nonaromatic Polycyclic 5-Iminooxazolidin-2-ones, Hydantoins, and Acylureas
- PMID: 28553003
- PMCID: PMC5444204
- DOI: 10.1016/j.tet.2008.02.033
Skeletal and Appendage Diversity as Design Elements in the Synthesis of a Discovery Library of Nonaromatic Polycyclic 5-Iminooxazolidin-2-ones, Hydantoins, and Acylureas
Abstract
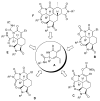
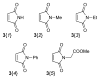
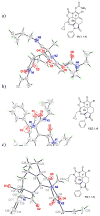
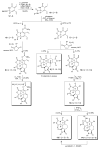
Amino acid-derived cross-conjugated trienes were used as a starting point for the synthesis of a discovery library of over 200 polycyclic 5-iminooxazolidin-2-ones, hydantoins, and acylureas. The main feature of this library synthesis is a triple branching strategy which provides efficient access to five skeletally diverse scaffolds. In addition, four sets of building blocks were applied in both a front end and a back end diversification strategy. Multiple fused rings were obtained by cyclization of diamides with phosgene and stereoselective Diels-Alder reactions with maleimides. The 5-iminooxazolidin-2-one scaffold was rearranged into the isomeric hydantoin scaffold through a sequence of ring opening and ring closing reactions.
Keywords: Appendage Diversity; Diels Alder Reaction; Library Design; Polycycles; Skeletal Diversity.
Figures
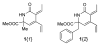
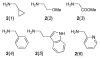
Similar articles
-
A diversity-oriented approach to spirocyclic and fused hydantoins via olefin metathesis.J Org Chem. 2012 Sep 21;77(18):8071-82. doi: 10.1021/jo301234r. Epub 2012 Sep 12. J Org Chem. 2012. PMID: 22938501
-
Rapid Access to Divergent Fused Polycycles Via One-Pot A3 Coupling and Intramolecular Diels-Alder Reaction.Chemistry. 2024 Jul 19;30(41):e202401449. doi: 10.1002/chem.202401449. Epub 2024 Jun 28. Chemistry. 2024. PMID: 38749918
-
Diversity-Oriented Approaches to Polycyclics and Bioinspired Molecules via the Diels-Alder Strategy: Green Chemistry, Synthetic Economy, and Beyond.ACS Comb Sci. 2015 May 11;17(5):253-302. doi: 10.1021/co500146u. Epub 2015 Apr 23. ACS Comb Sci. 2015. PMID: 25875156 Review.
-
Highly selective Diels-Alder and Heck arylation reactions in a divergent synthesis of isoindolo- and pyrrolo-fused polycyclic indoles from 2-formylpyrrole.Beilstein J Org Chem. 2020 Jun 17;16:1320-1334. doi: 10.3762/bjoc.16.113. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32595780 Free PMC article.
-
Design and Synthesis of Polycycles, Heterocycles, and Macrocycles via Strategic Utilization of Ring-Closing Metathesis.Chem Rec. 2018 Nov;18(11):1613-1632. doi: 10.1002/tcr.201800025. Epub 2018 Jun 19. Chem Rec. 2018. PMID: 29920922 Review.
Cited by
-
Design and synthesis of a library of tetracyclic hydroazulenoisoindoles.J Comb Chem. 2009 May-Jun;11(3):486-94. doi: 10.1021/cc900024p. J Comb Chem. 2009. PMID: 19366169 Free PMC article.
-
Untargeted Diversity-Oriented Synthesis for the Discovery of New Antitumor Agents: An Integrated Approach of Inverse Virtual Screening, Bioinformatics, and Omics for Target Deconvolution.J Med Chem. 2025 Aug 14;68(15):16483-16517. doi: 10.1021/acs.jmedchem.5c01344. Epub 2025 Jul 24. J Med Chem. 2025. PMID: 40705771 Free PMC article.
References
-
- Webb TR. Curr Opin Drug Disc Dev. 2005;8:303–308. - PubMed
-
- Schreiber SL. Science. 2000;287:1964–1969. - PubMed
- Burke MD, Lalic G. Chem Biol. 2002;9:535–541. - PubMed
- Spring DR. Org Biomol Chem. 2003;1:3867–3870. - PubMed
- Burke MD, Schreiber SL. Angew Chem Int Ed. 2004;43:46–58. - PubMed
- Taylor SJ, Taylor AM, Schreiber SL. Angew Chem Int Ed. 2004;43:1681–1685. - PubMed
-
- Tan DS. Nat Chem Biol. 2005;1:74–84. - PubMed
-
- Selzer P, Roth HJ, Ertl P, Schuffenhauer A. Curr Opin Chem Biol. 2005;9:310–316. - PubMed
-
- Liao Y, Hu Y, Wu J, Zhu Q, Donovan M, Fathi R, Yang Z. Curr Med Chem. 2003;10:2285–2316. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
