Dm5-HT2B: Pharmacological Characterization of the Fifth Serotonin Receptor Subtype of Drosophila melanogaster
- PMID: 28553207
- PMCID: PMC5425475
- DOI: 10.3389/fnsys.2017.00028
Dm5-HT2B: Pharmacological Characterization of the Fifth Serotonin Receptor Subtype of Drosophila melanogaster
Abstract
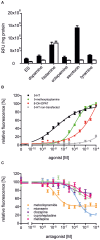
Serotonin (5-hydroxytryptamine, 5-HT) is an important regulator of physiological and behavioral processes in both protostomes (e.g., insects) and deuterostomes (e.g., mammals). In insects, serotonin has been found to modulate the heart rate and to control secretory processes, development, circadian rhythms, aggressive behavior, as well as to contribute to learning and memory. Serotonin exerts its activity by binding to and activating specific membrane receptors. The clear majority of these receptors belong to the superfamily of G-protein-coupled receptors. In Drosophila melanogaster, a total of five genes have been identified coding for 5-HT receptors. From this family of proteins, four have been pharmacologically examined in greater detail, so far. While Dm5-HT1A, Dm5-HT1B, and Dm5-HT7 couple to cAMP signaling cascades, the Dm5-HT2A receptor leads to Ca2+ signaling in an inositol-1,4,5-trisphosphate-dependent manner. Based on sequence similarity to homologous genes in other insects, a fifth D. melanogaster gene was uncovered coding for a Dm5-HT2B receptor. Knowledge about this receptor's pharmacological properties is very limited. This is quite surprising because Dm5-HT2B has been attributed to distinct physiological functions based on genetic interference with its gene expression. Mutations were described reducing the response of the larval heart to 5-HT, and specific knockdown of Dm5-HT2B mRNA in hemocytes resulted in a higher susceptibility of the flies to bacterial infection. To gain deeper understanding of Dm5-HT2B's pharmacology, we evaluated the receptor's response to a series of established 5-HT receptor agonists and antagonists in a functional cell-based assay. Metoclopramide and mianserin were identified as two potent antagonists that may allow pharmacological interference with Dm5-HT2B signaling in vitro and in vivo.
Keywords: 4; 5-trisphosphate; Ca2+; G protein-coupled receptor; biogenic amine; cAMP; cellular signaling; inositol-1; insect; second messenger.
Figures
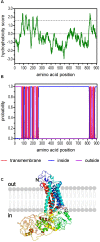

 ), PKA phosphorylation sites (
), PKA phosphorylation sites ( ), PKC phosphorylation sites (
), PKC phosphorylation sites ( ), phosphorylation sites for both PKA and PKC (
), phosphorylation sites for both PKA and PKC ( ), PKG phosphorylation sites (
), PKG phosphorylation sites ( ), phosphorylation sites for both PKA and PKG (
), phosphorylation sites for both PKA and PKG ( ), phosphorylation sites for all three kinases (
), phosphorylation sites for all three kinases ( ), and putative palmitoylation sites (∗) of Dm5-HT2B are indicated. The amino acid position is given on the right.
), and putative palmitoylation sites (∗) of Dm5-HT2B are indicated. The amino acid position is given on the right.
References
-
- Arakawa S., Gocayne J. D., McCombie W. R., Urquhart D. A., Hall L. M., Fraser C. M., et al. (1990). Cloning, localization, and permanent expression of a Drosophila octopamine receptor. Neuron 4 343–354. - PubMed
-
- Ballesteros J., Weinstein H. (1995). Integrated methods for the construction of three-dimensional models of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25 366–428.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

