The TOR Pathway Is Involved in Adventitious Root Formation in Arabidopsis and Potato
- PMID: 28553309
- PMCID: PMC5427086
- DOI: 10.3389/fpls.2017.00784
The TOR Pathway Is Involved in Adventitious Root Formation in Arabidopsis and Potato
Abstract
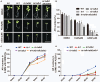
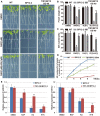
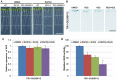
In the agriculture industry, adventitious root formation is a core issue of plants asexual propagation. However, the underlying molecular mechanism of adventitious root formation is far beyond understanding. In present study we found that target of rapamycin (TOR) signaling plays a key role in adventitious root formation in potato and Arabidopsis. The core components of TOR complex including TOR, RAPTOR, and LST8 are highly conserved in potato, but the seedlings of potato are insensitive to rapamycin, implying FK506 Binding Protein 12 KD (FKBP12) lost the function to bridge the interaction of rapamycin and TOR in potato. To dissect TOR signaling in potato, the rapamycin hypersensitive potato plants (BP12-OE) were engineered by introducing yeast FKBP12 (ScFKBP12) into potato. We found that rapamycin can significantly attenuate the capability of adventitious root formation in BP12-OE potatoes. KU63794 (KU, an active-site TOR inhibitor) combined with rapamycin can more significantly suppress adventitious root formation of BP12-OE potato than the single treatments, such as KU63794 or rapamycin, indicating its synergistic inhibitory effects on potato adventitious root formation. Furthermore, RNA-seq data showed that many genes associated with auxin signaling pathway were altered when BP12-OE potato seedlings were treated with rapamycin + KU, suggesting that TOR may play a major role in adventitious root formation via auxin signaling. The auxin receptor mutant tir1 was sensitive to TOR inhibitors and the double and quadruple mutants including tir1afb2, tir1afb3, and tir1afb1afb2afb3 displayed more sensitive to asTORis than single mutant tir1. Consistently, overexpression of AtTIR1 in Arabidopsis and potato can partially overcome the inhibitory effect of asTORis and promote adventitious root formation under asTORis treatments. These observations suggest that TOR signaling regulates adventitious root formation by mediating auxin signaling in Arabidopsis and potato.
Keywords: AtTIR1; adventitious root formation; auxin; potato; target of rapamycin.
Figures






Similar articles
-
Target of Rapamycin Is a Key Player for Auxin Signaling Transduction in Arabidopsis.Front Plant Sci. 2016 Mar 11;7:291. doi: 10.3389/fpls.2016.00291. eCollection 2016. Front Plant Sci. 2016. PMID: 27014314 Free PMC article.
-
Tomato FK506 Binding Protein 12KD (FKBP12) Mediates the Interaction between Rapamycin and Target of Rapamycin (TOR).Front Plant Sci. 2016 Nov 18;7:1746. doi: 10.3389/fpls.2016.01746. eCollection 2016. Front Plant Sci. 2016. PMID: 27917191 Free PMC article.
-
Brassinosteriod Insensitive 2 (BIN2) acts as a downstream effector of the Target of Rapamycin (TOR) signaling pathway to regulate photoautotrophic growth in Arabidopsis.New Phytol. 2017 Jan;213(1):233-249. doi: 10.1111/nph.14118. Epub 2016 Aug 1. New Phytol. 2017. PMID: 27479935
-
Auxin and Target of Rapamycin Spatiotemporally Regulate Root Organogenesis.Int J Mol Sci. 2021 Oct 21;22(21):11357. doi: 10.3390/ijms222111357. Int J Mol Sci. 2021. PMID: 34768785 Free PMC article. Review.
-
TOR inhibitors: from mammalian outcomes to pharmacogenetics in plants and algae.J Exp Bot. 2019 Apr 15;70(8):2297-2312. doi: 10.1093/jxb/erz053. J Exp Bot. 2019. PMID: 30773593 Review.
Cited by
-
Systemic control of plant regeneration and wound repair.New Phytol. 2023 Jan;237(2):408-413. doi: 10.1111/nph.18487. Epub 2022 Oct 2. New Phytol. 2023. PMID: 36101501 Free PMC article. Review.
-
Efficient regeneration of mature castanopsis hystrix from in vitro stem explants.Front Plant Sci. 2022 Aug 12;13:914652. doi: 10.3389/fpls.2022.914652. eCollection 2022. Front Plant Sci. 2022. PMID: 36035695 Free PMC article.
-
Comparisons between Plant and Animal Stem Cells Regarding Regeneration Potential and Application.Int J Mol Sci. 2023 Feb 23;24(5):4392. doi: 10.3390/ijms24054392. Int J Mol Sci. 2023. PMID: 36901821 Free PMC article. Review.
-
Molecular and physiological control of adventitious rooting in cuttings: phytohormone action meets resource allocation.Ann Bot. 2019 Jun 24;123(6):929-949. doi: 10.1093/aob/mcy234. Ann Bot. 2019. PMID: 30759178 Free PMC article.
-
The plant TOR kinase tunes autophagy and meristem activity for nutrient stress-induced developmental plasticity.Plant Cell. 2022 Sep 27;34(10):3814-3829. doi: 10.1093/plcell/koac201. Plant Cell. 2022. PMID: 35792878 Free PMC article.
References
-
- Agulló-Antón M. Á., Sánchez-Bravo J., Acosta M., Druege U. (2010). Auxins or sugars: what makes the difference in the adventitious rooting of stored carnation cuttings? J. Plant Growth Regul. 30, 100–113. 10.1007/s00344-010-9174-8 - DOI
-
- Amissah N., Akakpo B., Yeboah J., Blay E. (2013). Asexual propagation of sheanut tree (C.F. Gaertn.) using a container layering technique. Am. J. Plant Sci. 4, 1758–1764. 10.4236/ajps.2013.49216 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

