Non-coding RNAs in the Ovarian Follicle
- PMID: 28553318
- PMCID: PMC5427069
- DOI: 10.3389/fgene.2017.00057
Non-coding RNAs in the Ovarian Follicle
Abstract
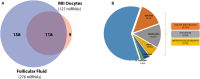
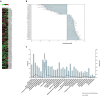
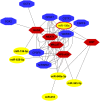
The mammalian ovarian follicle is the complex reproductive unit comprising germ cell, somatic cells (Cumulus and Granulosa cells), and follicular fluid (FF): paracrine communication among the different cell types through FF ensures the development of a mature oocyte ready for fertilization. This paper is focused on non-coding RNAs in ovarian follicles and their predicted role in the pathways involved in oocyte growth and maturation. We determined the expression profiles of microRNAs in human oocytes and FF by high-throughput analysis and identified 267 microRNAs in FF and 176 in oocytes. Most of these were FF microRNAs, while 9 were oocyte specific. By bioinformatic analysis, independently performed on FF and oocyte microRNAs, we identified the most significant Biological Processes and the pathways regulated by their validated targets. We found many pathways shared between the two compartments and some specific for oocyte microRNAs. Moreover, we found 41 long non-coding RNAs able to interact with oocyte microRNAs and potentially involved in the regulation of folliculogenesis. These data are important in basic reproductive research and could also be useful for clinical applications. In fact, the characterization of non-coding RNAs in ovarian follicles could improve reproductive disease diagnosis, provide biomarkers of oocyte quality in Assisted Reproductive Treatment, and allow the development of therapies for infertility disorders.
Keywords: follicular fluid; human oocyte; lncRNAs; microRNAs; ovarian follicle.
Figures

References
-
- Bartel D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116 281–297. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

