Axon degeneration: make the Schwann cell great again
- PMID: 28553320
- PMCID: PMC5436338
- DOI: 10.4103/1673-5374.205000
Axon degeneration: make the Schwann cell great again
Abstract
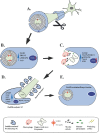
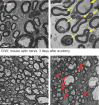
Axonal degeneration is a pivotal feature of many neurodegenerative conditions and substantially accounts for neurological morbidity. A widely used experimental model to study the mechanisms of axonal degeneration is Wallerian degeneration (WD), which occurs after acute axonal injury. In the peripheral nervous system (PNS), WD is characterized by swift dismantling and clearance of injured axons with their myelin sheaths. This is a prerequisite for successful axonal regeneration. In the central nervous system (CNS), WD is much slower, which significantly contributes to failed axonal regeneration. Although it is well-documented that Schwann cells (SCs) have a critical role in the regenerative potential of the PNS, to date we have only scarce knowledge as to how SCs 'sense' axonal injury and immediately respond to it. In this regard, it remains unknown as to whether SCs play the role of a passive bystander or an active director during the execution of the highly orchestrated disintegration program of axons. Older reports, together with more recent studies, suggest that SCs mount dynamic injury responses minutes after axonal injury, long before axonal breakdown occurs. The swift SC response to axonal injury could play either a pro-degenerative role, or alternatively a supportive role, to the integrity of distressed axons that have not yet committed to degenerate. Indeed, supporting the latter concept, recent findings in a chronic PNS neurodegeneration model indicate that deactivation of a key molecule promoting SC injury responses exacerbates axonal loss. If this holds true in a broader spectrum of conditions, it may provide the grounds for the development of new glia-centric therapeutic approaches to counteract axonal loss.
Keywords: Wallerian degeneration; dedifferentiation; glia; myelin; neurodegeneration; oligodendrocytes.
Figures
References
-
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

