Human Adipose-Derived Stem Cells Exhibit Enhanced Proliferative Capacity and Retain Multipotency Longer than Donor-Matched Bone Marrow Mesenchymal Stem Cells during Expansion In Vitro
- PMID: 28553357
- PMCID: PMC5434475
- DOI: 10.1155/2017/2541275
Human Adipose-Derived Stem Cells Exhibit Enhanced Proliferative Capacity and Retain Multipotency Longer than Donor-Matched Bone Marrow Mesenchymal Stem Cells during Expansion In Vitro
Abstract
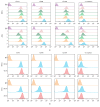
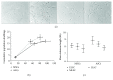
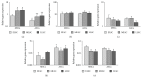
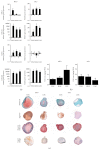
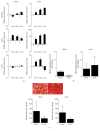
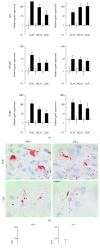
Bone marrow-derived mesenchymal stem cells (MSCs) and adipose-derived multipotent/mesenchymal stem cells (ASCs) have been proposed as the ideal cell types for a range of musculoskeletal tissue engineering and regenerative medicine therapies. However, extensive in vitro expansion is required to generate sufficient cells for clinical application and previous studies have demonstrated differences in the proliferative capacity and the impact of expansion on differentiation capacity of both MSCs and ASCs. Significantly, these studies routinely use cells from different donors, making direct comparisons difficult. Importantly, this study directly compared the proliferative capacity and multipotency of human MSCs and ASCs from the same donors to determine how each cell type was affected by in vitro expansion. The study identified that ASCs were able to proliferate faster and undergo greater population doublings than donor-matched MSCs and that senescence was primarily driven via telomere shortening and upregulation of p16ink4a. Both donor-matched MSCs and ASCs were capable of trilineage differentiation early in cultures; however, while differentiation capacity diminished with time in culture, ASCs retained enhanced capacity compared to MSCs. These findings suggest that ASCs may be the most appropriate cell type for musculoskeletal tissue engineering and regenerative medicine therapies due to their enhanced in vitro expansion capacity and limited loss of differentiation potential.
Figures
References
-
- Ashton B. A., Allen T. D., Howlett C. R., Eaglesom C. C., Hattori A., Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clinical Orthopaedics and Related Research. 1980;151(151):294–307. - PubMed
-
- Friedenstein A. J., Chailakhyan R. K., Gerasimov U. V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell and Tissue Kinetics. 1987;20(3):263–272. - PubMed
-
- Friedenstein A. J., Deriglasova U. F., Kulagina N. N., et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Experimental Hematology. 1974;2(2):83–92. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

