Crystal Structure of the Superconducting Phase of Sulfur Hydride
- PMID: 28553364
- PMCID: PMC5446087
- DOI: 10.1038/nphys3760
Crystal Structure of the Superconducting Phase of Sulfur Hydride
Abstract
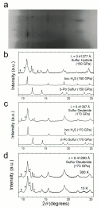
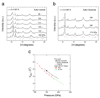
A superconducting critical temperature above 200 K has recently been discovered in H2S (or D2S) under high hydrostatic pressure1, 2. These measurements were interpreted in terms of a decomposition of these materials into elemental sulfur and a hydrogen-rich hydride that is responsible for the superconductivity, although direct experimental evidence for this mechanism has so far been lacking. Here we report the crystal structure of the superconducting phase of hydrogen sulfide (and deuterium sulfide) in the normal and superconducting states obtained by means of synchrotron X-ray diffraction measurements, combined with electrical resistance measurements at both room and low temperatures. We find that the superconducting phase is mostly in good agreement with theoretically predicted body-centered cubic (bcc) structure for H3S (Ref.3). The presence of elemental sulfur is also manifest in the X-ray diffraction patterns, thus proving the decomposition mechanism of H2S to H3S + S under pressure4-6.
Conflict of interest statement
Competing financial interests The authors declare that they have no competing financial interests.
Figures

References
-
- Drozdov AP, Eremets MI, Troyan IA. Conventional superconductivity at 190 K at high pressures. 2014 Preprint at http://arxiv.org/abs/1412.0460. - PubMed
-
- Drozdov AP, Eremets MI, Troyan IA, Ksenofontov V, Shylin SI. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature. 2015;525:73–77. - PubMed
-
- Bernstein N, Hellberg CS, Johannes MD, Mazin II, Mehl MJ. What superconducts in sulfur hydrides under pressure, and why. Phys Rev B. 2015;91:060511(R).
-
- Duan D, et al. Pressure-induced decomposition of solid hydrogen sulfide. Phys Rev B. 2015;91:180502(R).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
