Sleep Disturbance During Smoking Cessation: Withdrawal or Side Effect of Treatment?
- PMID: 28553407
- PMCID: PMC5443250
- DOI: 10.1017/jsc.2016.11
Sleep Disturbance During Smoking Cessation: Withdrawal or Side Effect of Treatment?
Abstract
Introduction: The nicotine-metabolite ratio (NMR) predicts treatment response and is related to treatment side effect severity. Sleep disturbance may be one important side effect, but understanding sleep disturbance effects on smoking cessation is complicated by the fact that nicotine withdrawal also produces sleep disturbance.
Aims: To evaluate the effects of withdrawal and treatment side effects on sleep disturbance.
Methods: This is a secondary analysis of data from a clinical trial (Lerman et al., 2015) of 1,136 smokers randomised to placebo (n = 363), transdermal nicotine (TN; n = 381), or varenicline (n = 392) and stratified based on NMR (559 slow metabolisers; 577 normal metabolisers). Sleep disturbance was assessed at baseline and at 1-week following the target quit date (TQD). We also examined whether sleep disturbance predicted 7-day point-prevalence abstinence at end-of-treatment (EOT).
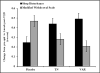
Results: The varenicline and TN groups exhibited greater increases in sleep disturbance (vs. placebo; treatment × time interaction; p = 0.005), particularly among those who quit smoking at 1-week post-TQD. There was a main effect of NMR (p = 0.04), but no interactions with treatment. TN and varenicline attenuated withdrawal symptoms unrelated to sleep (vs. placebo). Greater baseline sleep disturbance predicted relapse at EOT (p = 0.004).
Conclusions: Existing treatments may not mitigate withdrawal-related sleep disturbance and adjunctive treatments that target sleep disturbance may improve abstinence rates.
Conflict of interest statement
Conflict of Interest CL received study medication and placebo, and support for medication packaging, from Pfizer; she has also consulted to Gilead, and has been a paid expert witness in litigation against tobacco companies. PC served on the scientific advisory board of Pfizer Pharmaceuticals, did educational talks sponsored by Pfizer on smoking cessation from 2006 to 2008, and has received grant support from Pfizer. RAS received medication and placebo free of charge from Pfizer for a different project, and has consulted to Pfizer and GlaxoSmithKline. TPG has had both investigator-initiated and industry-sponsored grants from Pfizer in the past 12 months, and serves on a data monitoring committee for Novartis. RFT has acted as a consultant to pharmaceutical companies, primarily on smoking cessation. The remaining authors declare no competing interests.
Figures


References
-
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12(2):101–112.
-
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
