Macrocycles: lessons from the distant past, recent developments, and future directions
- PMID: 28553456
- PMCID: PMC5424464
- DOI: 10.1039/c4sc03089c
Macrocycles: lessons from the distant past, recent developments, and future directions
Abstract
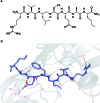
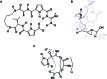
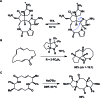
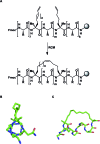
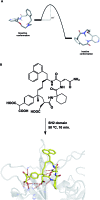
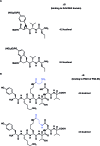
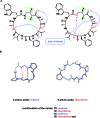
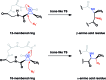
A noticeable increase in molecular complexity of drug targets has created an unmet need in the therapeutic agents that are larger than traditional small molecules. Macrocycles, which are cyclic compounds comprising 12 atoms or more, are now recognized as molecules that "are up to the task" to interrogate extended protein interfaces. However, because macrocycles (particularly the ones based on peptides) are equipped with large polar surface areas, achieving cellular permeability and bioavailability is anything but straightforward. While one might consider this to be the Achilles' heel of this class of compounds, the synthetic community continues to develop creative approaches toward the synthesis of macrocycles and their site-selective modification. This perspective provides an overview of both mechanistic and structural issues that bear on macrocycles as a unique class of molecules. The reader is offered a historical foray into some of the classic studies that have resulted in the current renaissance of macrocycles. In addition, an attempt is made to overview the more recent developments that give hope that macrocycles might indeed turn into a useful therapeutic modality.
Figures












References
-
- Frank A. T., Farina N. S., Sawwan N., Wauchope O. R., Qi M., Brzostowska E. M., Chan W., Grasso F. W., Haberfield P., Greer A. Mol. Diversity. 2007;11:115–118. - PubMed
-
- Wessjohann L. A., Ruijter E., Garcia-Rivera D., Brandt W. Mol. Diversity. 2005;9:171–186. - PubMed
-
- Pedersen C. J. Angew. Chem., Int. Ed. Engl. 1988;27:1021–1027.
-
- Madala P. K., Tyndall J. D. A., Nall T., Fairlie D. P. Chem. Rev. 2010;110:PR1–PR31. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

