Ultrafast delocalization of excitation in synthetic light-harvesting nanorings
- PMID: 28553466
- PMCID: PMC5424671
- DOI: 10.1039/c4sc02424a
Ultrafast delocalization of excitation in synthetic light-harvesting nanorings
Abstract
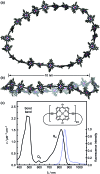
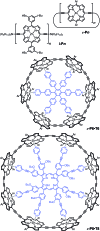
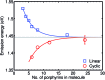
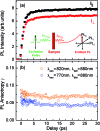
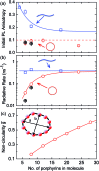
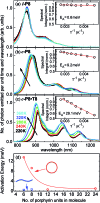
Rings of chlorophyll molecules harvest sunlight remarkably efficiently during photosynthesis in purple bacteria. The key to their efficiency lies in their highly delocalized excited states that allow for ultrafast energy migration. Here we show that a family of synthetic nanorings mimic the ultrafast energy transfer and delocalization observed in nature. π-Conjugated nanorings with diameters of up to 10 nm, consisting of up to 24 porphyrin units, are found to exhibit excitation delocalization within the first 200 fs of light absorption. Transitions from the first singlet excited state of the circular nanorings are dipole-forbidden as a result of symmetry constraints, but these selection rules can be lifted through static and dynamic distortions of the rings. The increase in the radiative emission rate in the larger nanorings correlates with an increase in static disorder expected from Monte Carlo simulations. For highly symmetric rings, the radiative rate is found to increase with increasing temperature. Although this type of thermally activated superradiance has been theoretically predicted in circular chromophore arrays, it has not previously been observed in any natural or synthetic systems. As expected, the activation energy for emission increases when a nanoring is fixed in a circular conformation by coordination to a radial template. These nanorings offer extended chromophores with high excitation delocalization that is remarkably stable against thermally induced disorder. Such findings open new opportunities for exploring coherence effects in nanometer molecular rings and for implementing these biomimetic light-harvesters in man-made devices.
Figures

References
-
- Cogdell R. J., Gall A., Köhler J. Q. Rev. Biophys. 2006;39:227. - PubMed
-
- McDermott G., Prince S. M., Freer A. A., Hawthornthwaite-Lawless A. M., Papiz M. Z., Cogdell R. J., Isaacs N. W. Nature. 1995;374:517. - PubMed
-
- van Oijen A. M., Ketelaars M., Köhler J., Aartsma T. J., Schmidt J. Science. 1999;285:400. - PubMed
-
- Engel G. S., Calhoun T. R., Read E. L., Ahn T.-K., Mančal T., Cheng Y.-C., Blankenship R. E., Fleming G. R. Nature. 2007;446:782. - PubMed
-
- Herek J. L., Wohlleben W., Cogdell R. J., Zeidler D., Motzkus M. Nature. 2002;417:533. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

