Visualization of drug delivery processes using AIEgens
- PMID: 28553485
- PMCID: PMC5431685
- DOI: 10.1039/c6sc05421h
Visualization of drug delivery processes using AIEgens
Abstract
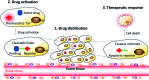
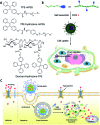
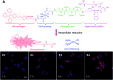
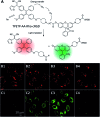
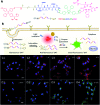
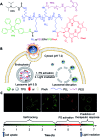
Drug delivery systems (DDSs) have been extensively studied as carriers to deliver small molecule chemo-drugs to tumors for cancer therapy. The therapeutic efficiency of chemo-drugs is crucially dependent on the effective drug concentrations in tumors and cancer cells. Novel DDSs that can simultaneously unveil drug distribution, drug release/activation behaviors and offer early evaluation of their therapeutic responses are highly desirable. Traditional fluorescent dye-labeled DDSs may suffer from notorious aggregation-caused quenching (ACQ) with limited sensitivity for bioimaging; in addition, the intrinsic fluorescence of these dyes requires careful selection of energy acceptor or quencher moieties for a light-up probe design, which complicates the development of self-reporting DDSs, especially the ones for reporting multiple processes. The recently emerged fluorogens with aggregation-induced emission characteristics (AIEgens) offer a straightforward solution to tackle this challenge. Thanks to the unique properties of AIEgens, new theranostic DDSs have been developed for simultaneous drug delivery and bioimaging with high signal to background ratio and multiple signal reporting capabilities. In this mini-review, we summarize the recent development of theranostic DDSs based on AIEgens for monitoring the drug distribution, drug activation and prediction of the therapeutic responses. Through illustration of their design principles and application examples, we hope to stimulate the interest in the design of more advanced theranostic DDSs for biomedical research.
Figures
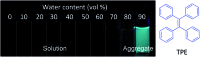






Similar articles
-
AIEgen based drug delivery systems for cancer therapy.J Control Release. 2018 Nov 28;290:129-137. doi: 10.1016/j.jconrel.2018.09.028. Epub 2018 Oct 5. J Control Release. 2018. PMID: 30296460 Review.
-
Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights.Acc Chem Res. 2018 Jun 19;51(6):1404-1414. doi: 10.1021/acs.accounts.8b00060. Epub 2018 May 7. Acc Chem Res. 2018. PMID: 29733571
-
Specific light-up bioprobes based on AIEgen conjugates.Chem Soc Rev. 2015 May 21;44(10):2798-811. doi: 10.1039/c4cs00444b. Epub 2015 Feb 17. Chem Soc Rev. 2015. PMID: 25686761 Review.
-
Recent Advances in Metal-Organic Frameworks as Anticancer Drug Delivery Systems: A Review.Anticancer Agents Med Chem. 2021;21(18):2487-2504. doi: 10.2174/1871520621666210119093844. Anticancer Agents Med Chem. 2021. PMID: 33463479 Review.
-
Aggregation-Induced Emission Luminogens for Activity-Based Sensing.Acc Chem Res. 2019 Sep 17;52(9):2559-2570. doi: 10.1021/acs.accounts.9b00305. Epub 2019 Aug 22. Acc Chem Res. 2019. PMID: 31436083 Review.
Cited by
-
Consequences of a cosolvent on the structure and molecular dynamics of supramolecular polymers in water.Chem Sci. 2018 Jun 11;9(29):6199-6209. doi: 10.1039/c8sc02257g. eCollection 2018 Aug 7. Chem Sci. 2018. PMID: 30090307 Free PMC article.
-
An AIEgen-based 3D covalent organic framework for white light-emitting diodes.Nat Commun. 2018 Dec 7;9(1):5234. doi: 10.1038/s41467-018-07670-4. Nat Commun. 2018. PMID: 30532031 Free PMC article.
-
Nanoparticle-based Cell Trackers for Biomedical Applications.Theranostics. 2020 Jan 12;10(4):1923-1947. doi: 10.7150/thno.39915. eCollection 2020. Theranostics. 2020. PMID: 32042345 Free PMC article. Review.
-
Exploring the trans-membrane dynamic mechanisms of single polyamidoamine nano-drugs via a "force tracing" technique.RSC Adv. 2018 Feb 27;8(16):8626-8630. doi: 10.1039/c8ra00134k. eCollection 2018 Feb 23. RSC Adv. 2018. PMID: 35539864 Free PMC article.
-
On the encapsulation and assembly of anticancer drugs in a cooperative fashion.Chem Sci. 2019 May 8;10(22):5678-5685. doi: 10.1039/c9sc01380f. eCollection 2019 Jun 14. Chem Sci. 2019. PMID: 31293752 Free PMC article.
References
-
- Rosen H., Abribat T. Nat. Rev. Drug Discovery. 2005;4:381. - PubMed
-
- Langer R. Nature. 1998;392:5. - PubMed
-
- Tong R., Tang L., Ma L., Tu C., Baumgartner R., Cheng J. Chem. Soc. Rev. 2014;43:6982. - PubMed
-
- Yoo J. W., Irvine D. J., Discher D. E., Mitragotri S. Nat. Rev. Drug Discovery. 2011;10:521. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

