Cobalt-catalysed C-H carbonylative cyclisation of aliphatic amides
- PMID: 28553492
- PMCID: PMC5431632
- DOI: 10.1039/c6sc05581h
Cobalt-catalysed C-H carbonylative cyclisation of aliphatic amides
Abstract
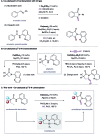
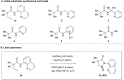
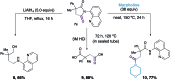
A cobalt-catalysed C-H carbonylation of aliphatic carboxamide derivatives is described, employing commercially available Co(ii)-salts in the presence of a silver oxidant. This operationally simple process utilises an atmospheric pressure of CO and generates a range of substituted succinimide products bearing diverse functional groups that can be successfully accessed via this methodology.
Figures
References
-
- Yamaguchi J., Yamaguchi A. D., Itami K. Angew. Chem., Int. Ed. 2012;51:8960–9009. - PubMed
- Lyons T. W., Sanford M. S. Chem. Rev. 2010;110:1147–1169. - PMC - PubMed
- Mkhalid I. A. I., Barnard J. H., Marder T. B., Murphy J. M., Hartwig J. F. Chem. Rev. 2010;110:890–931. - PubMed
- Wencel-Delord J., Dröge T., Liu F., Glorius F. Chem. Soc. Rev. 2011;40:4740–4761. - PubMed
- Jazzar R., Hitce J., Renaudat A., Sofack-Kreutzer J., Baudoin O. Chemistry. 2010;16:2654–2672. - PubMed
- Zhu R.-Y., Farmer M. E., Chen Y.-Q., Yu J. Q. Angew. Chem., Int. Ed. 2016;55:10578–10599. - PMC - PubMed
- Gao K., Yoshikai N. Acc. Chem. Res. 2014;47:1208. - PubMed
- Colby D. A., Bergmann R. G., Ellman J. A. Chem. Rev. 2010;110:624–655. - PMC - PubMed
-
-
Selected recent reviews on first row transition metal catalysed C-H bond functionalization see:
- Wei D., Zhu X., Niu J.-L., Song M.-P. ChemCatChem. 2016;8:1242–1263.
- Moselage N., Li J., Ackermann L. ACS Catal. 2016;6:498–525.
- Yoshikai N. ChemCatChem. 2015;7:732–734.
- Hyster T. Catal. Lett. 2015;145:458–467.
- Bauer I., Knölker H. Chem. Rev. 2015;115:3170–3387. - PubMed
- Tasker S. Z., Standley E. A., Jamison T. F. Nature. 2014;509:299–309. - PMC - PubMed
- Kulkarni A. A., Daugulis O. Synthesis. 2009:4087–4109.
-
-
- Murahashi S. J. Am. Chem. Soc. 1955;77:6403–6404.
-
-
For selected references on high valent cobalt catalyzed C(sp3)–H bond functionalization see:
- Prakash S., Muralirajan K., Cheng C.-H. Angew. Chem., Int. Ed. 2016;55:1844–1848. - PubMed
- Du C., Li P.-X., Zhu X., Suo J.-F., Niu J.-L., Song M.-P. Angew. Chem., Int. Ed. 2016;55:13571–13575. - PubMed
- Maity S., Kancherla R., Dhawa U., Hoque E., Pimparkar S., Maiti D. ACS Catal. 2016;6:5493–5499.
- Lerchen A., Vásquez-Céspedes S., Glorius F. Angew. Chem., Int. Ed. 2016;55:3208–3211. - PubMed
- Tan G., He S., Huang X., Liao X., Cheng Y., You J. Angew. Chem., Int. Ed. 2016;55:10414–10418. - PubMed
- Manoharan R., Sivakumar G., Jeganmohan M. Chem. Commun. 2016;52:10533–10536. - PubMed
- Yamaguchi T., Kommagalla Y., Aihara Y., Chatani N. Chem. Commun. 2016;52:10129–10132. - PubMed
- Kong L., Yu S., Zhou X., Li X. Org. Lett. 2016;18:588–591. - PubMed
- Hummel J. R., Ellman J. A. J. Am. Chem. Soc. 2015;137:490–498. - PMC - PubMed
- Patel P., Chang S. ACS Catal. 2015;5:853–858.
- Wang H., Koeller J., Liu W., Ackermann L. Chem.–Eur. J. 2015;21:15525–15528. - PubMed
- Zhang Z.-Z., Bin L., Wang C.-Y., Shi B.-F. Org. Lett. 2015;17:4094–4097. - PubMed
- Yoshino T., Ikemoto H., Matsunaga S., Kanai M. Angew. Chem., Int. Ed. 2013;52:2207–2211. - PubMed
- Grigorjeva L., Daugulis O. Org. Lett. 2014;16:4688–4690. - PMC - PubMed
-
-
- Zaitsev V. G., Shabashov D., Daugulis O. J. Am. Chem. Soc. 2005;127:13154–13155. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources