A smart ZnO@polydopamine-nucleic acid nanosystem for ultrasensitive live cell mRNA imaging by the target-triggered intracellular self-assembly of active DNAzyme nanostructures
- PMID: 28553521
- PMCID: PMC5427684
- DOI: 10.1039/c6sc04633a
A smart ZnO@polydopamine-nucleic acid nanosystem for ultrasensitive live cell mRNA imaging by the target-triggered intracellular self-assembly of active DNAzyme nanostructures
Abstract
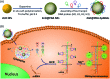
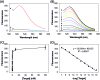
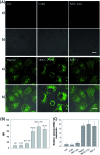
Efficient strategies for the ultrasensitive imaging of gene expression in living cells are essential in chemistry and cell biology. Here, we report a novel and efficient enzyme-free dual signal amplification strategy for live cell mRNA imaging by using a smart nucleic acid hairpin-based nanosystem. This nanosystem consists of a ZnO nanoparticle core, an interlayer of polydopamine and an outer layer of four hairpin DNA (hpDNA) probes. Such a core-shell nanosystem facilitates the cellular uptake of molecular hairpin payloads, protects them from nuclease digestion, and delivers them into the cytoplasm by the acid-triggered dissolution of the ZnO core. In the presence of target mRNA, the released hpDNA probes self-assemble via HCR into wire-shaped active DNAzymes that catalyze the generation of a fluorescence signal. The target-initiated HCR events and DNAzyme cascades offer efficient dual amplification and enable the ultrasensitive detection of mRNA with a femtomolar detection limit. Live cell assays show an intense fluorescence response from a tumor-related biomarker survivin mRNA only in tumor cells untreated with a survivin expression repressor YM155, but not in normal cells. The developed nanosystem provides a potential platform for the amplified imaging of low-abundance disease-related biomarkers in live cells.
Figures



Similar articles
-
Smart Hairpins@MnO2 Nanosystem Enables Target-Triggered Enzyme-Free Exponential Amplification for Ultrasensitive Imaging of Intracellular MicroRNAs in Living Cells.Anal Chem. 2022 Jun 7;94(22):8014-8023. doi: 10.1021/acs.analchem.2c01211. Epub 2022 May 20. Anal Chem. 2022. PMID: 35594196
-
Electrostatic nucleic acid nanoassembly enables hybridization chain reaction in living cells for ultrasensitive mRNA imaging.J Am Chem Soc. 2015 Jun 3;137(21):6829-36. doi: 10.1021/jacs.5b01778. Epub 2015 May 20. J Am Chem Soc. 2015. PMID: 25969953
-
A biodegradable and cofactor self-sufficient aptazyme nanoprobe for amplified imaging of low-abundance protein in living cells.Talanta. 2023 Feb 1;253:123983. doi: 10.1016/j.talanta.2022.123983. Epub 2022 Oct 1. Talanta. 2023. PMID: 36201958
-
DNAzyme-Amplified Cascade Catalytic Hairpin Assembly Nanosystem for Sensitive MicroRNA Imaging in Living Cells.Anal Chem. 2023 Aug 8;95(31):11793-11799. doi: 10.1021/acs.analchem.3c02071. Epub 2023 Jul 4. Anal Chem. 2023. PMID: 37402285
-
Functional Nucleic Acid-Based Live-Cell Fluorescence Imaging.Front Chem. 2020 Dec 11;8:598013. doi: 10.3389/fchem.2020.598013. eCollection 2020. Front Chem. 2020. PMID: 33363111 Free PMC article. Review.
Cited by
-
Detection of intracellular microRNA-21 for cancer diagnosis by a nanosystem containing a ZnO@polydopamine and DNAzyme probe.RSC Adv. 2024 Apr 26;14(19):13351-13360. doi: 10.1039/d4ra00636d. eCollection 2024 Apr 22. RSC Adv. 2024. PMID: 38680416 Free PMC article.
-
A DNAzyme-amplified DNA circuit for highly accurate microRNA detection and intracellular imaging.Chem Sci. 2019 Aug 26;10(41):9597-9604. doi: 10.1039/c9sc03552d. eCollection 2019 Nov 7. Chem Sci. 2019. PMID: 32055333 Free PMC article.
-
Biomineralized metal-organic framework nanoparticles enable a primer exchange reaction-based DNA machine to work in living cells for imaging and gene therapy.Chem Sci. 2020 Jun 16;11(27):7092-7101. doi: 10.1039/d0sc00339e. eCollection 2020 Jul 21. Chem Sci. 2020. PMID: 33250978 Free PMC article.
-
DNAzyme-functionalized porous carbon nanospheres serve as a fluorescent nanoprobe for imaging detection of microRNA-21 and zinc ion in living cells.Mikrochim Acta. 2020 Mar 27;187(4):249. doi: 10.1007/s00604-020-04226-6. Mikrochim Acta. 2020. PMID: 32221723
-
Nanoparticles modified by polydopamine: Working as "drug" carriers.Bioact Mater. 2020 Apr 18;5(3):522-541. doi: 10.1016/j.bioactmat.2020.04.003. eCollection 2020 Sep. Bioact Mater. 2020. PMID: 32322763 Free PMC article. Review.
References
-
- Wills Q. F., Livak K. J., Tipping A. J., Enver T., Goldson A. J., Sexton D. W., Holmes C. Nat. Biotechnol. 2013;31:748. - PubMed
- Chattopadhyay P. K., Gierahn T. M., Roederer M., Love J. C. Nat. Immunol. 2014;15:128. - PMC - PubMed
- Zhao X., Xu L., Sun M., Ma W., Wu X., Kuang H., Wang L., Xu C. Small. 2016;12:4662. - PubMed
- Xu L., Zhao S., Ma W., Wu X., Li S., Kuang H., Wang L., Xu C. Adv. Funct. Mater. 2016;26:1602.
- Li S., Xu L., Ma W., Wu X., Sun M., Kuang H., Wang L., Kotov N. A., Xu C. J. Am. Chem. Soc. 2016;138:306. - PubMed
-
- Huang J., Wang H., Yang X., Yang Y., Quan K., Ying L., Xie N., Ou M., Wang K. Chem. Commun. 2016;52:370. - PubMed
-
- Cheglakov Z., Cronin T. M., He C., Weizmann Y. J. Am. Chem. Soc. 2015;137:6116. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

