A smart ZnO@polydopamine-nucleic acid nanosystem for ultrasensitive live cell mRNA imaging by the target-triggered intracellular self-assembly of active DNAzyme nanostructures
- PMID: 28553521
- PMCID: PMC5427684
- DOI: 10.1039/c6sc04633a
A smart ZnO@polydopamine-nucleic acid nanosystem for ultrasensitive live cell mRNA imaging by the target-triggered intracellular self-assembly of active DNAzyme nanostructures
Abstract
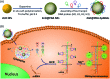
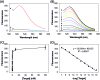
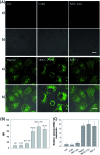
Efficient strategies for the ultrasensitive imaging of gene expression in living cells are essential in chemistry and cell biology. Here, we report a novel and efficient enzyme-free dual signal amplification strategy for live cell mRNA imaging by using a smart nucleic acid hairpin-based nanosystem. This nanosystem consists of a ZnO nanoparticle core, an interlayer of polydopamine and an outer layer of four hairpin DNA (hpDNA) probes. Such a core-shell nanosystem facilitates the cellular uptake of molecular hairpin payloads, protects them from nuclease digestion, and delivers them into the cytoplasm by the acid-triggered dissolution of the ZnO core. In the presence of target mRNA, the released hpDNA probes self-assemble via HCR into wire-shaped active DNAzymes that catalyze the generation of a fluorescence signal. The target-initiated HCR events and DNAzyme cascades offer efficient dual amplification and enable the ultrasensitive detection of mRNA with a femtomolar detection limit. Live cell assays show an intense fluorescence response from a tumor-related biomarker survivin mRNA only in tumor cells untreated with a survivin expression repressor YM155, but not in normal cells. The developed nanosystem provides a potential platform for the amplified imaging of low-abundance disease-related biomarkers in live cells.
Figures



References
-
- Wills Q. F., Livak K. J., Tipping A. J., Enver T., Goldson A. J., Sexton D. W., Holmes C. Nat. Biotechnol. 2013;31:748. - PubMed
- Chattopadhyay P. K., Gierahn T. M., Roederer M., Love J. C. Nat. Immunol. 2014;15:128. - PMC - PubMed
- Zhao X., Xu L., Sun M., Ma W., Wu X., Kuang H., Wang L., Xu C. Small. 2016;12:4662. - PubMed
- Xu L., Zhao S., Ma W., Wu X., Li S., Kuang H., Wang L., Xu C. Adv. Funct. Mater. 2016;26:1602.
- Li S., Xu L., Ma W., Wu X., Sun M., Kuang H., Wang L., Kotov N. A., Xu C. J. Am. Chem. Soc. 2016;138:306. - PubMed
-
- Huang J., Wang H., Yang X., Yang Y., Quan K., Ying L., Xie N., Ou M., Wang K. Chem. Commun. 2016;52:370. - PubMed
-
- Cheglakov Z., Cronin T. M., He C., Weizmann Y. J. Am. Chem. Soc. 2015;137:6116. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

