Determination of a complex crystal structure in the absence of single crystals: analysis of powder X-ray diffraction data, guided by solid-state NMR and periodic DFT calculations, reveals a new 2'-deoxyguanosine structural motif
- PMID: 28553539
- PMCID: PMC5433513
- DOI: 10.1039/c7sc00587c
Determination of a complex crystal structure in the absence of single crystals: analysis of powder X-ray diffraction data, guided by solid-state NMR and periodic DFT calculations, reveals a new 2'-deoxyguanosine structural motif
Abstract
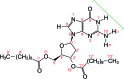
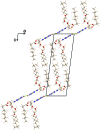
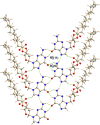
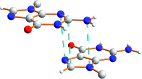
Derivatives of guanine exhibit diverse supramolecular chemistry, with a variety of distinct hydrogen-bonding motifs reported in the solid state, including ribbons and quartets, which resemble the G-quadruplex found in nucleic acids with sequences rich in guanine. Reflecting this diversity, the solid-state structural properties of 3',5'-bis-O-decanoyl-2'-deoxyguanosine, reported in this paper, reveal a hydrogen-bonded guanine ribbon motif that has not been observed previously for 2'-deoxyguanosine derivatives. In this case, structure determination was carried out directly from powder XRD data, representing one of the most challenging organic molecular structures (a 90-atom molecule) that has been solved to date by this technique. While specific challenges were encountered in the structure determination process, a successful outcome was achieved by augmenting the powder XRD analysis with information derived from solid-state NMR data and with dispersion-corrected periodic DFT calculations for structure optimization. The synergy of experimental and computational methodologies demonstrated in the present work is likely to be an essential feature of strategies to further expand the application of powder XRD as a technique for structure determination of organic molecular materials of even greater complexity in the future.
Figures

Similar articles
-
Leucopterin, the white pigment in butterfly wings: structural analysis by PDF fit, FIDEL fit, Rietveld refinement, solid-state NMR and DFT-D.IUCrJ. 2023 Jul 1;10(Pt 4):448-463. doi: 10.1107/S2052252523004281. IUCrJ. 2023. PMID: 37335768 Free PMC article.
-
Exploiting the Synergy of Powder X-ray Diffraction and Solid-State NMR Spectroscopy in Structure Determination of Organic Molecular Solids.J Phys Chem C Nanomater Interfaces. 2013 Jun 13;117(23):12258-12265. doi: 10.1021/jp4041106. Epub 2013 May 3. J Phys Chem C Nanomater Interfaces. 2013. PMID: 24386493 Free PMC article.
-
Probing hydrogen bonding and ion-carbonyl interactions by solid-state 17O NMR spectroscopy: G-ribbon and G-quartet.J Am Chem Soc. 2007 Feb 28;129(8):2398-407. doi: 10.1021/ja067991m. Epub 2007 Feb 2. J Am Chem Soc. 2007. PMID: 17269776
-
Powder diffraction crystallography of molecular solids.Top Curr Chem. 2012;315:133-77. doi: 10.1007/128_2011_251. Top Curr Chem. 2012. PMID: 21952843 Review.
-
NMR crystallography of molecular organics.Prog Nucl Magn Reson Spectrosc. 2020 Jun-Aug;118-119:10-53. doi: 10.1016/j.pnmrs.2020.03.001. Epub 2020 Mar 19. Prog Nucl Magn Reson Spectrosc. 2020. PMID: 32883448 Review.
Cited by
-
Ambiguous structure determination from powder data: four different structural models of 4,11-di-fluoro-quinacridone with similar X-ray powder patterns, fit to the PDF, SSNMR and DFT-D.IUCrJ. 2022 May 14;9(Pt 4):406-424. doi: 10.1107/S2052252522004237. eCollection 2022 Jul 1. IUCrJ. 2022. PMID: 35844476 Free PMC article.
-
A structure determination protocol based on combined analysis of 3D-ED data, powder XRD data, solid-state NMR data and DFT-D calculations reveals the structure of a new polymorph of l-tyrosine.Chem Sci. 2022 Mar 30;13(18):5277-5288. doi: 10.1039/d1sc06467c. eCollection 2022 May 11. Chem Sci. 2022. PMID: 35655549 Free PMC article.
-
Enantiotropy of Simvastatin as a Result of Weakened Interactions in the Crystal Lattice: Entropy-Driven Double Transitions and the Transient Modulated Phase as Seen by Solid-State NMR Spectroscopy.Molecules. 2022 Jan 20;27(3):679. doi: 10.3390/molecules27030679. Molecules. 2022. PMID: 35163943 Free PMC article.
-
Manometric real-time studies of the mechanochemical synthesis of zeolitic imidazolate frameworks.Chem Sci. 2020 Jan 2;11(8):2141-2147. doi: 10.1039/c9sc05514b. Chem Sci. 2020. PMID: 34123303 Free PMC article.
-
Unveiling the topology of partially disordered micro-crystalline nitro-perylenediimide with X-aggregate stacking: an integrated approach.Chem Sci. 2023 Nov 23;15(2):490-499. doi: 10.1039/d3sc05514k. eCollection 2024 Jan 3. Chem Sci. 2023. PMID: 38179523 Free PMC article.
References
-
- Lightfoot P., Tremayne M., Harris K. D. M., Bruce P. G. J. Chem. Soc., Chem. Commun. 1992:1012–1013.
-
- Harris K. D. M., Tremayne M., Lightfoot P., Bruce P. G. J. Am. Chem. Soc. 1994;116:3543–3547.
-
- Chernyshev V. V. Russ. Chem. Bull. 2001;50:2273–2292.
-
- Harris K. D. M., Tremayne M., Kariuki B. M. Angew. Chem., Int. Ed. 2001;40:1626–1651. - PubMed
-
- Structure Determination from Powder Diffraction Data, ed. W. I. F. David, K. Shankland, L. B. McCusker and C. Baerlocher, IUCr/OUP, 2002. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

